Question: please show all work i will rate The table shows the variation of the rate constant with temperature for the first-order reaction. 2N2O5(g)2N2O4(g)+O2(g) Determine graphically
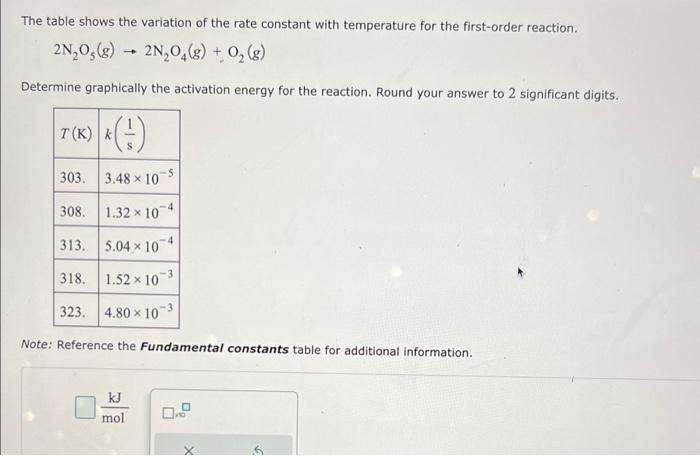
The table shows the variation of the rate constant with temperature for the first-order reaction. 2N2O5(g)2N2O4(g)+O2(g) Determine graphically the activation energy for the reaction. Round your answer to 2 significant digits. Note: Reference the Fundamental constants table for additional information. molkJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


