Question: please show all work thanks 14. Write two balanced half-reactions for the following redox reaction. Fe(g) + Cu(s) + Cu(aq) + Fe2(ma). 15 Balance using
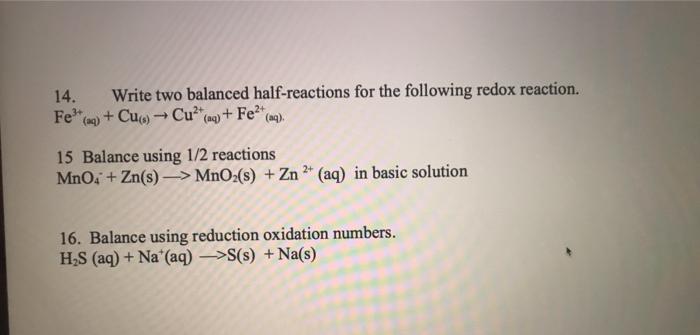
14. Write two balanced half-reactions for the following redox reaction. Fe(g) + Cu(s) + Cu(aq) + Fe2(ma). 15 Balance using 1/2 reactions MnO4 + Zn(s) -> MnO2(s) + Zn + (aq) in basic solution 16. Balance using reduction oxidation numbers. HAS (aq) + Na+ (aq)->S(8) + Na(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


