Question: please show all your work for these three questions 9. The dimerization of cyclopentadiene (CH) occurs in a simple one-step elementary process as shown below:
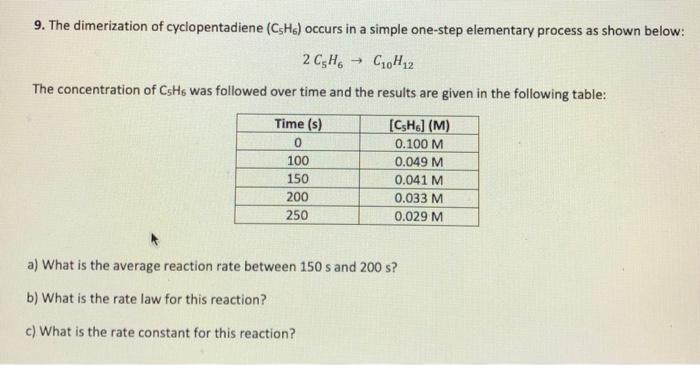


9. The dimerization of cyclopentadiene (CH) occurs in a simple one-step elementary process as shown below: 2C5H C10H 2 The concentration of CsHs was followed over time and the results are given in the following table: Time (s) 0 100 150 200 250 [CH] (M) 0.100 M 0.049 M 0.041 M 0.033 M 0.029 M a) What is the average reaction rate between 150 s and 200 s? b) What is the rate law for this reaction? c) What is the rate constant for this reaction? 5. The rate constant for a first order reaction is 4.60 x 104s1 at 350C. If the activation energy is 104 kJ mol, calculate the temperature at which its rate constant is 5.28 x 102 min1. Watch your units! 11. Catalase is an important enzyme that breaks down its substrate, H202, into water and oxygen in nearly all living cells. The enzyme has kz = 1.1*10' secand km = 2.0 mM. An experiment was set up under the conditions when the reaction rate is independent of (H2O2). In this experiment, at what concentration of catalase (use SI units and scientific notation) will the reaction have a rate of 850 mm/min
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


