Question: please show full solution (5) The critical temperature and pression of CO2 are To=304.2K and pe=72.8 atm, respectively. Assuming that the gas can be described
please show full solution
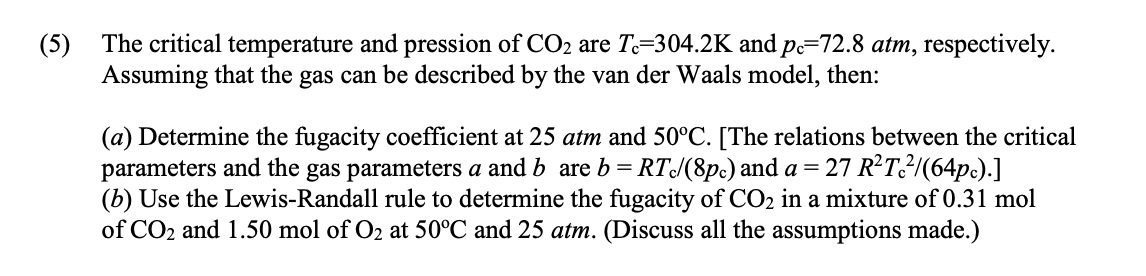
(5) The critical temperature and pression of CO2 are To=304.2K and pe=72.8 atm, respectively. Assuming that the gas can be described by the van der Waals model, then: = (a) Determine the fugacity coefficient at 25 atm and 50C. [The relations between the critical parameters and the gas parameters a and b are b= RT/(8pc) and a= 27 RT_2/(64pc).] (b) Use the Lewis-Randall rule to determine the fugacity of CO2 in a mixture of 0.31 mol of CO2 and 1.50 mol of O2 at 50C and 25 atm. (Discuss all the assumptions made.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


