Question: please show how to do number 3 and 4 What is the molarity of a solution made by dissolving 37.4gFe(NO3)3 in enough water to make
please show how to do number 3 and 4 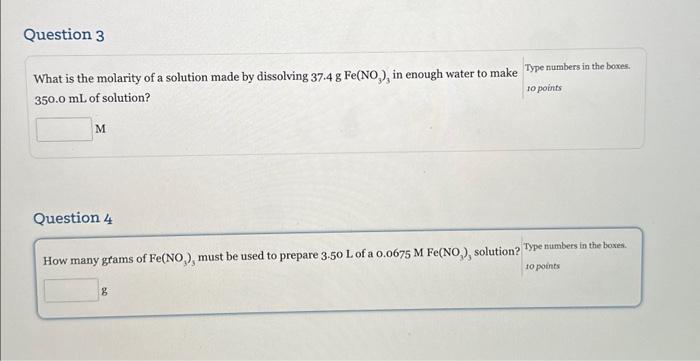

What is the molarity of a solution made by dissolving 37.4gFe(NO3)3 in enough water to make 350.0mL of solution? to points M Question 4 How many grams of Fe(NO3)3 must be used to prepare 3.50Lofa0.0675MFeNO)3 solution? Type numbers in the boxes. to points g
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


