Question: Please show how you do it in as much detail as possible! Thank you! 2. Vapor Pressures of solutions a. Non-volatile solute - 18 molal
Please show how you do it in as much detail as possible! Thank you!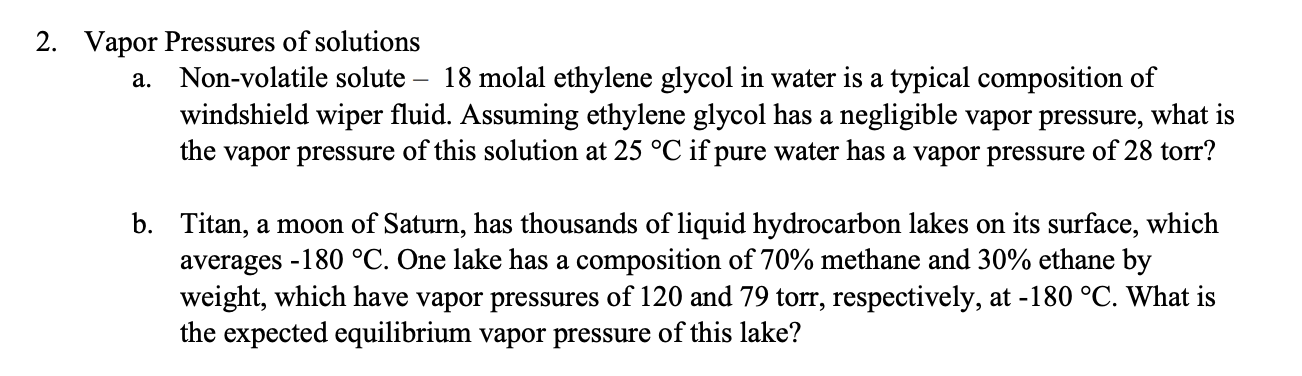
2. Vapor Pressures of solutions a. Non-volatile solute - 18 molal ethylene glycol in water is a typical composition of windshield wiper fluid. Assuming ethylene glycol has a negligible vapor pressure, what is the vapor pressure of this solution at 25C if pure water has a vapor pressure of 28 torr? b. Titan, a moon of Saturn, has thousands of liquid hydrocarbon lakes on its surface, which averages 180C. One lake has a composition of 70% methane and 30% ethane by weight, which have vapor pressures of 120 and 79 torr, respectively, at 180C. What is the expected equilibrium vapor pressure of this lake
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


