Question: please show me how to do this with all calculations of how you get 1/T and lnK and the excel graph. remember it has to

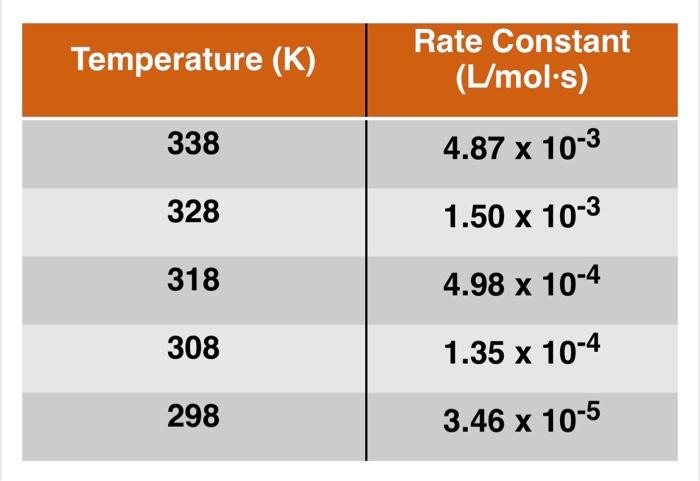
Each student will receive their own, unique data set via email. Answer the following questions concerning the data set given using MS Excel. The Excel file should contain your data and a proper graph. Insert a text box to answer questions that require an answer in words. The Excel file must be uploaded in Canvas for grading by the deadline. The linear form (y = mx + b) of the Arrhenius equation is given below: Ink = a + InA -Ea + RT 1. In order to find the activation energy (E.) you will need to make a linear graph. What should be plotted on the y-axis? The x-axis? 2. Will the slope of such a line be equal to E,? If not, write an equation relating the slope (m) to E. 3. Using the variable b to represent the y-intercept, write an equation relating b to A, the frequency factor 4. Make an Arrhenius plot in MS Excel using the data you were given and determine the values of E, and A. Be sure that the graph you hand in is labeled and titled properly. 5. What is the value of R for this data? What does it mean? Temperature (K) Rate Constant (L/mol.s) 338 4.87 x 10-3 328 1.50 x 10-3 318 4.98 x 10-4 308 1.35 x 10-4 298 3.46 x 10-5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


