Question: please show step by step answer to this question. thankyou lots The joule (J) is a unit of energy. Recall that energy may be converted

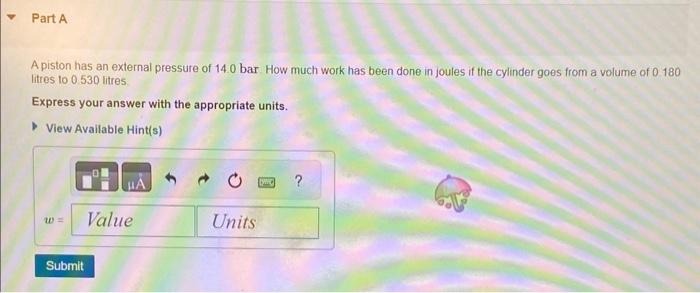
The joule (J) is a unit of energy. Recall that energy may be converted between many different forms such as mechanical energy, thermal energy (heat), chemical energy, electrical energy, and light. The mechanical energy produced by a system is called work. When work is accomplished through the changing volume of a gas, it is called PV work and is given by the formula w=PV, where w is the . work, P is the external pressure, and V is the change in volume. If the volume change and pressure are in litres and bars respectively, then the work will have units of litre-bars, which can be converted to joules using the conversion factor 1L bar =100J. Work can also be expressed as force multiplied by distance: w=Fd, where w is the work in joules, F is the force in newtons, and d is the distance in metres. Note that 1Nm=1J. A piston has an external pressure of 14.0 bar How much work has been done in joules if the cylinder goes from a volume of 0.180 litres to 0.530 litres Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


