Question: Please show steps . 8) For CH3COOH(aq) + H200 = CH3COO(aq) + H2O(aq); K = 1.8-10-5 at 25C. What would you expect the equilibrium concentration
Please show steps .
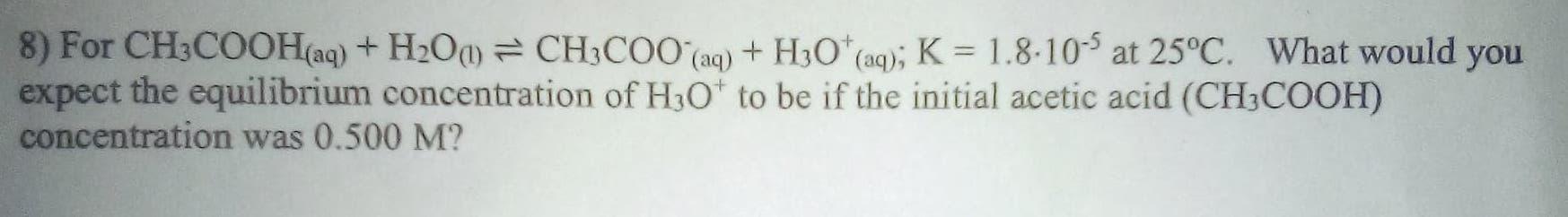
8) For CH3COOH(aq) + H200 = CH3COO(aq) + H2O(aq); K = 1.8-10-5 at 25C. What would you expect the equilibrium concentration of H3O+ to be if the initial acetic acid (CH3COOH) concentration was 0.500 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


