Question: Please show the steps. Thank you! 2. Using partial charges (+/), indicate the net polarity (+) of the following molecules below: (a) (b) (c) 3.
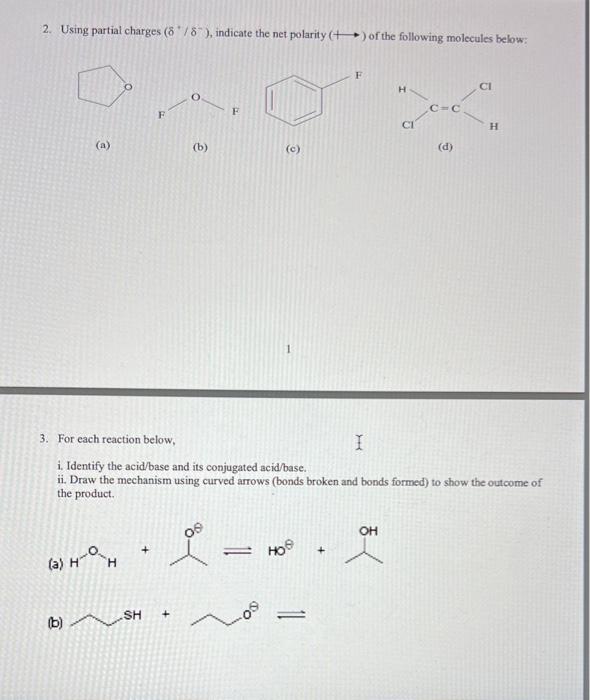
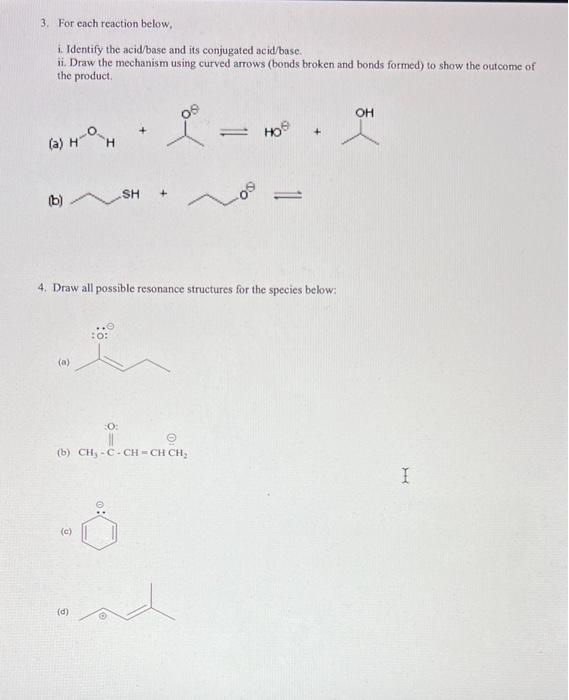
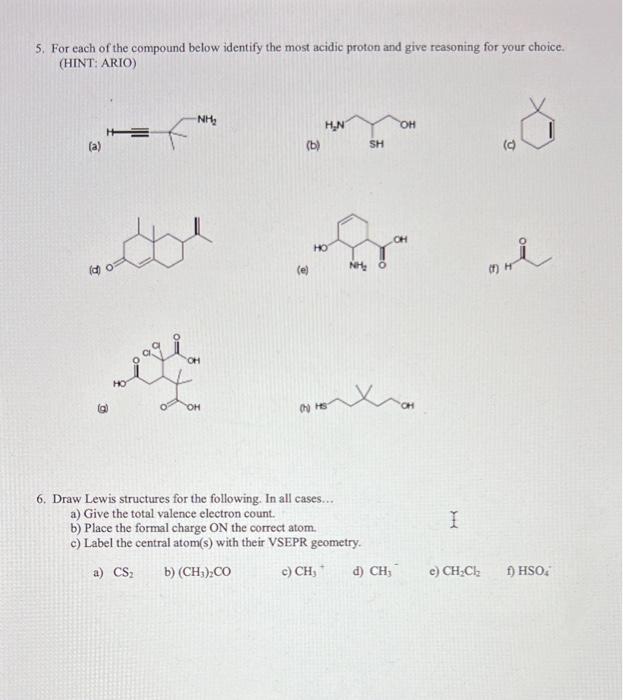
2. Using partial charges (+/), indicate the net polarity (+) of the following molecules below: (a) (b) (c) 3. For each reaction below, i. Identify the acid/base and its conjugated acid/base. ii. Draw the mechanism using curved arrows (bonds broken and bonds formed) to show the outcome of the product. (a) H0 (b) 3. For each reaction below, i. Identify the acid/base and its conjugated acid/base. ii. Draw the mechanism using curved arrows (bonds broken and bonds formed) to show the outcome of the product. (a) HO6 (b) 4. Draw all possible resonance structures for the species below: (a) (b) (c) (d) 5. For each of the compound below identify the most acidic proton and give reasoning for your choice. (HINT: ARIO) (a) (b) (d) (e) (f) (a) 6. Draw Lewis structures for the following. In all cases... a) Give the total valence electron count. b) Place the formal charge ON the correct atom. c) Label the central atom(s) with their VSEPR geometry. a) CS2 b) (CH3)2CO c) CH3 d) CH3 e) CH2Cl2 f) HSO4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


