Question: please show with number Density of Water as a Function of Temperature Image Credit: https://pressbooks.nscc.ca/chemistryatoms/back-motter/water-properties/ The graph above shows that the density of water decreases
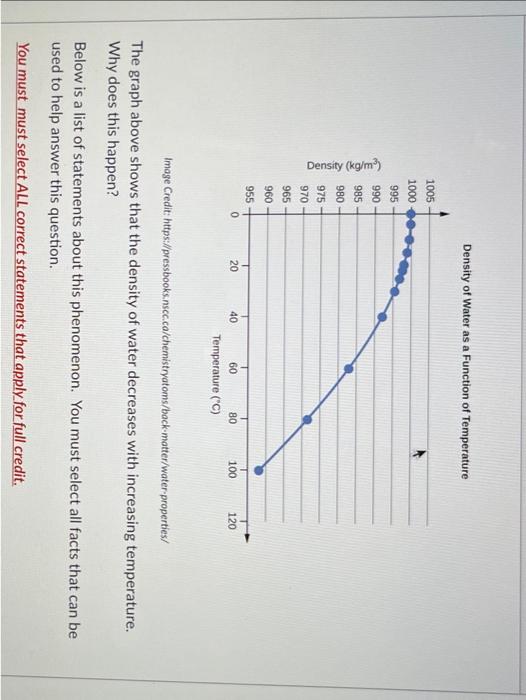

Density of Water as a Function of Temperature Image Credit: https://pressbooks.nscc.ca/chemistryatoms/back-motter/water-properties/ The graph above shows that the density of water decreases with increasing temperature. Why does this happen? Below is a list of statements about this phenomenon. You must select all facts that can be used to help answer this question. You must must select ALL correct statements that apply for full credit. You must must select ALL correct statements that apply for full credit. hotter means greater kinetic energy hotter means faster higher kinetic energy causes particles to push one another farther apart for the same mass, increasing kinetic energy results in greater volume for the same mass, density increases with increasing volume for the equation "a=c/b", "a" decreases when "b" increases, given "c" is constant higher kinetic energy causes particles to push one another closer together hotter means lower kinetic energy for the same mass, increasing kinetic energy results in lower volume hotter means slower for the same mass, density decreases with increasing volume for the equation "a=c/b", "a" increases when "b" increases, given "c" is constant Density of Water as a Function of Temperature Image Credit: https://pressbooks.nscc.ca/chemistryatoms/back-motter/water-properties/ The graph above shows that the density of water decreases with increasing temperature. Why does this happen? Below is a list of statements about this phenomenon. You must select all facts that can be used to help answer this question. You must must select ALL correct statements that apply for full credit. You must must select ALL correct statements that apply for full credit. hotter means greater kinetic energy hotter means faster higher kinetic energy causes particles to push one another farther apart for the same mass, increasing kinetic energy results in greater volume for the same mass, density increases with increasing volume for the equation "a=c/b", "a" decreases when "b" increases, given "c" is constant higher kinetic energy causes particles to push one another closer together hotter means lower kinetic energy for the same mass, increasing kinetic energy results in lower volume hotter means slower for the same mass, density decreases with increasing volume for the equation "a=c/b", "a" increases when "b" increases, given "c" is constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


