Question: Please show work! 2. What is the order for each reactant and the overall rate law for the following reaction (leave the rate constant as
Please show work!
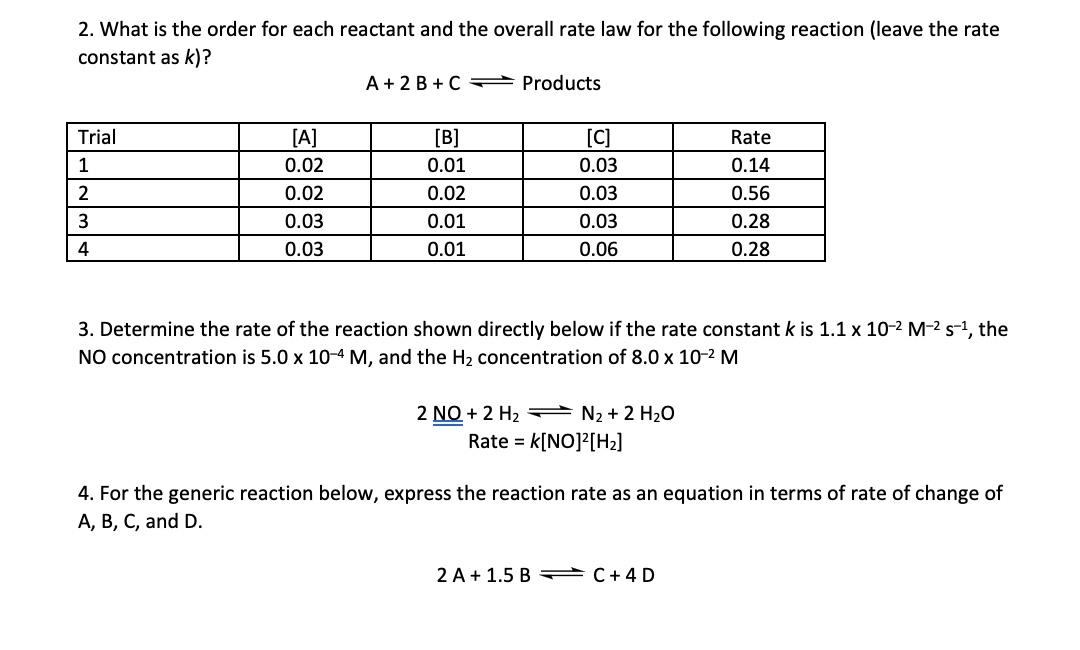
2. What is the order for each reactant and the overall rate law for the following reaction (leave the rate constant as k) ? A+2B+CProducts 3. Determine the rate of the reaction shown directly below if the rate constant k is 1.1102M2s1, the NO concentration is 5.0104M, and the H2 concentration of 8.0102M 2NO+2H2N2+2H2ORate=k[NO]2[H2] 4. For the generic reaction below, express the reaction rate as an equation in terms of rate of change of A,B,C, and D. 2A+1.5BC+4D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


