Question: please answer all Short Answer - You may use separate paper it you need more space 1 whether the overall reaction is endothermic or exothermic

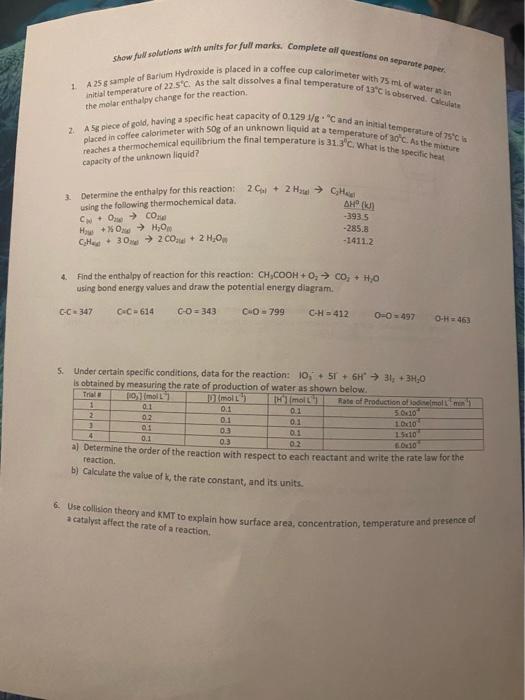
Short Answer - You may use separate paper it you need more space 1 whether the overall reaction is endothermic or exothermic and how you can determine this. 5 Briefly explain what is being shown at each letter of the following diagram. Also, be sure to state Ep B E 2. In the classroom setting coffee cup calorimeters are very useful in exploring systems with small enere changes. However, some experimental assumptions must be made when using these calorimeters. Explain what these assumptions are 3. The enthalpy of formation for Benzene, CH is 49.0 l/mol. Represent this thermochemical reaction in three different ways. Show fint solutions with units for full marks. Complete all questions on separate paper 1 A 25 sample of Barium Hydroxide is placed in a coffee cup calorimeter with 75 ml of water in initial temperature of 22.5C. As the salt dissolves a final temperature of 13" is observed. Cal 2A Splece of gold, having a specific heat capacity of 0.129 1/8"Cand an initial temperature of SC is placed in coffee calorimeter with sop of an unknown liquid at a temperature of 30C. As the more reaches a thermochemical equilibrium the final temperature is 31.3C. What is the specifiche the molar enthalpy change for the reaction capacity of the unknown liquid? Determine the enthalpy for this reaction: using the following thermochemical data COCO How + 0 Hj0 Chee. 302C+ 2 H, 2.0 + 2 Hal CHE AH -3935 -285.8 -14112 4. Find the enthalpy of reaction for this reaction: CH.COOH + 0, CO, HO using bond energy values and draw the potential energy diagram. C-C347 G-C=614 C-D=343 C.O-799 C-H = 412 O = 497 O- H463 5. Under certain specific conditions, data for the reaction: 10, + 5 + 6H 31; +34,0 is obtained by measuring the rate of production of water as shown below. Trial moll moll Hmolt Rate of Production of a molt 1 0.1 0.1 0.1 5.000 2 02 0.1 0.1 1.00 1 01 oa 0.1 4 15:10 0.1 0.3 02 000 a) Determine the order of the reaction with respect to each reactant and write the rate law for the reaction. b) Calculate the value of the rate constant, and its units. 6. Use collision theory and KMT to explain how surface area, concentration, temperature and presence of a catalyst affect the rate of a reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


