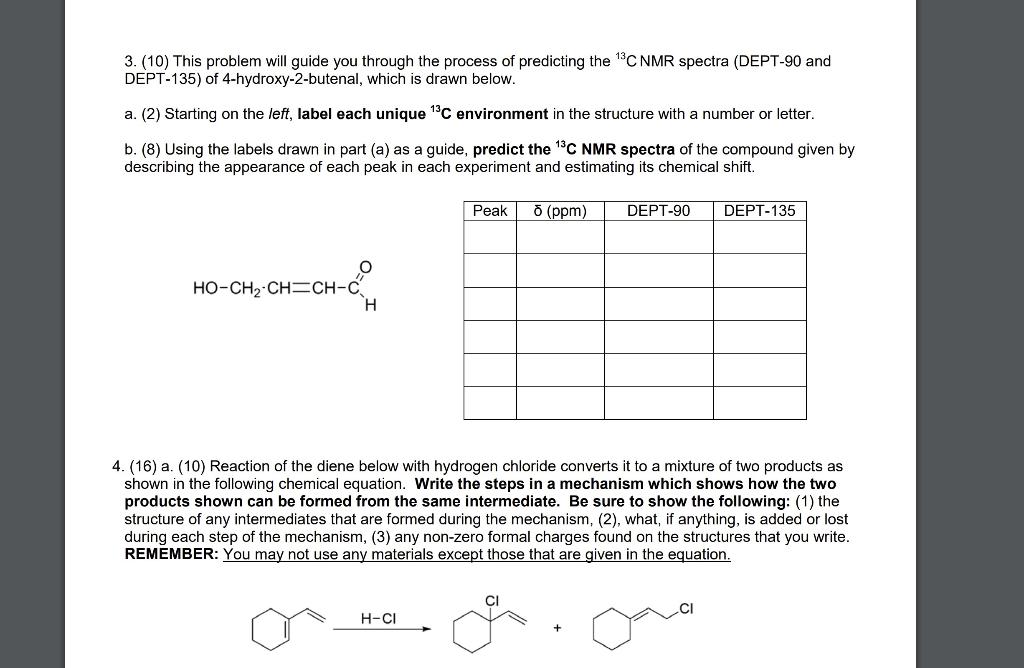
Question: Please show work 3. (10) This problem will guide you through the process of predicting the 13CNMR spectra (DEPT-90 and DEPT-135) of 4-hydroxy-2-butenal, which is
Please show work
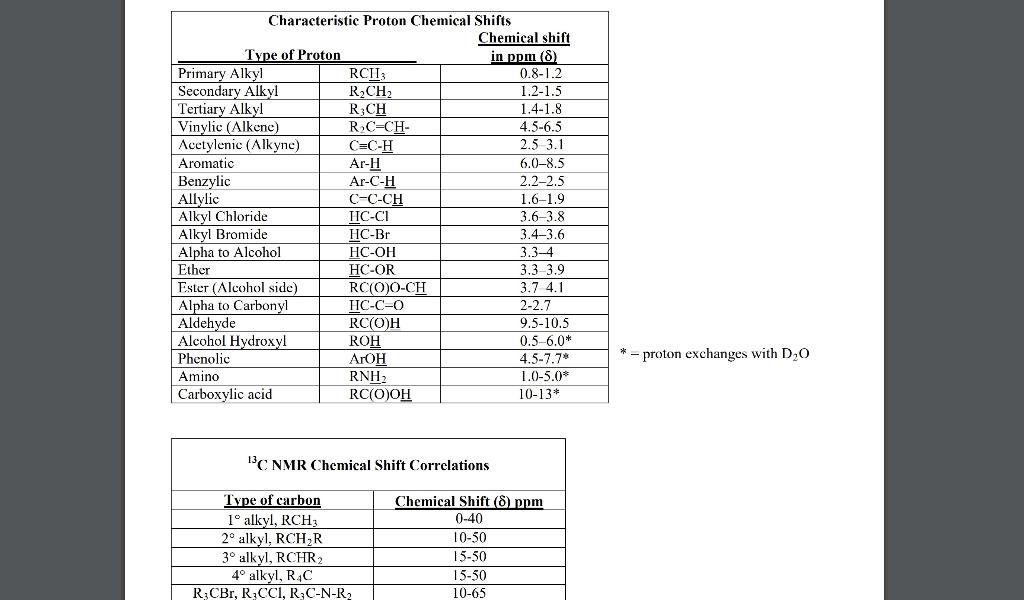
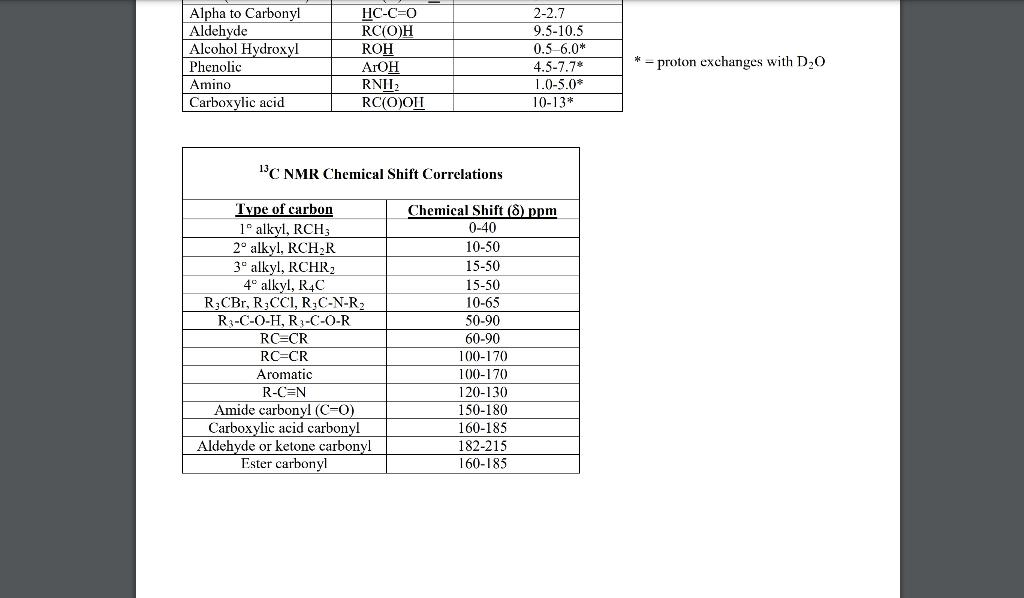
3. (10) This problem will guide you through the process of predicting the 13CNMR spectra (DEPT-90 and DEPT-135) of 4-hydroxy-2-butenal, which is drawn below. a. (2) Starting on the left, label each unique 13C environment in the structure with a number or letter. b. (8) Using the labels drawn in part (a) as a guide, predict the 13CNMR spectra of the compound given by describing the appearance of each peak in each experiment and estimating its chemical shift. 4. (16) a. (10) Reaction of the diene below with hydrogen chloride converts it to a mixture of two products as shown in the following chemical equation. Write the steps in a mechanism which shows how the two products shown can be formed from the same intermediate. Be sure to show the following: (1) the structure of any intermediates that are formed during the mechanism, (2), what, if anything, is added or lost during each step of the mechanism, (3) any non-zero formal charges found on the structures that you write. REMEMBER: You may not use any materials except those that are given in the equation. \begin{tabular}{|l|l|c|} \hline \multicolumn{2}{|c|}{ Characteristic Proton Chemical Shifts } \\ \multicolumn{2}{|c|}{ Chemical shift } \\ Type of Proton \end{tabular} 13 C. NMR Chemical Shift Correlations = proton exchanges with D2O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


