Question: please show work and do all parts if possible ! 1. Calculate the volume (mL) of concentrated H2SO4 needed to produce 1 L of 0.2N
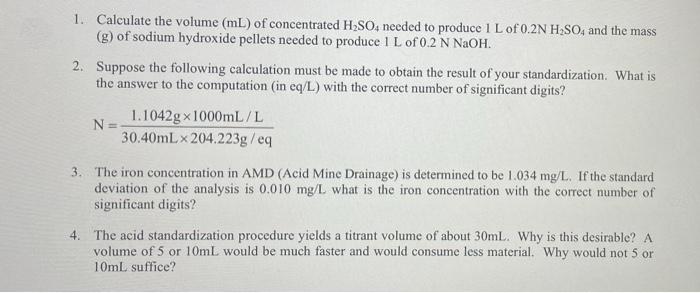
1. Calculate the volume (mL) of concentrated H2SO4 needed to produce 1 L of 0.2N H2SO4 and the mass (g) of sodium hydroxide pellets needed to produce 1 L of 0.2 N NaOH. 2. Suppose the following calculation must be made to obtain the result of your standardization. What is the answer to the computation in eq/L) with the correct number of significant digits? 1.1042g x 1000mL/L N= 30.40mL x 204.223g/eq 3. The iron concentration in AMD (Acid Mine Drainage) is determined to be 1.034 mg/L. If the standard deviation of the analysis is 0.010 mg/L what is the iron concentration with the correct number of significant digits? 4. The acid standardization procedure yields a titrant volume of about 30mL. Why is this desirable? A volume of 5 or 10mL would be much faster and would consume less material. Why would not 5 or 10mL suffice
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


