Question: Please show work, my answers are incorrect and need help A magnesium-lead alloy of mass 8.0 kg consists of a solid a phase that has
Please show work, my answers are incorrect and need help
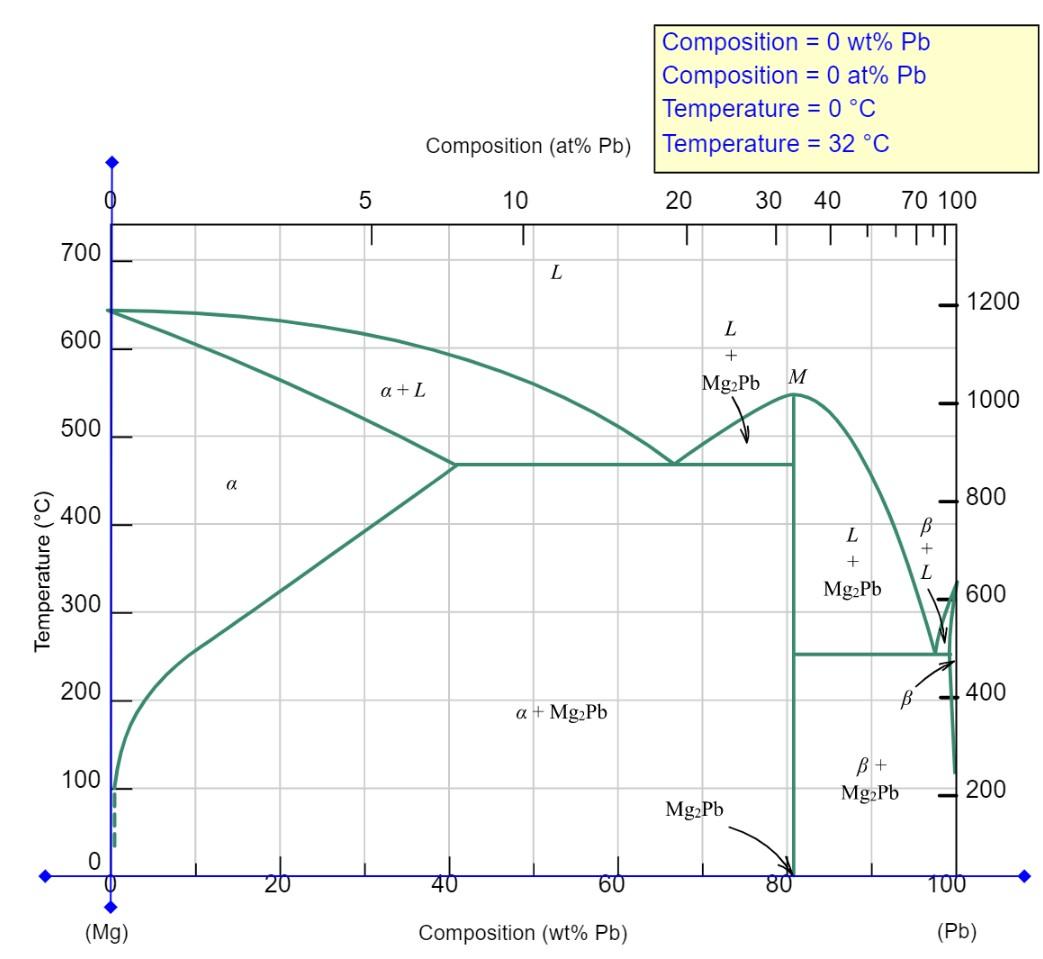
A magnesium-lead alloy of mass 8.0 kg consists of a solid a phase that has a composition just slightly below the solubility limit at 300C (570F). The magnesium-lead phase diagram is shown in Animated Figure 9.20. (a) What mass of lead is in the alloy? i 1.496 kg (b) If the alloy is heated to 400C (750F), how much more lead may be dissolved in the a phase without exceeding the solubility limit of this phase? 1.9411 kg Composition = 0 wt% Pb Composition = 0 at% Pb Temperature = 0 C Composition (at% Pb) Temperature = 32 C - 5 10 20 30 40 70 100 700 L 1200 600 L + 1 M a+L Mg2Pb 1000 500 a 800 400 L B + L + Temperature (C) Mg2Pb 600 300 200 - 400 a + Mg2Pb 100 Mg2Pb 200 Mg2Pb 0 60 80 100 (Mg) Composition (wt% Pb) (Pb)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


