Question: Please show work to prove this. A heat engine is reported to take in 150kW of heat at a temperature of 600C and convert it
Please show work to prove this.
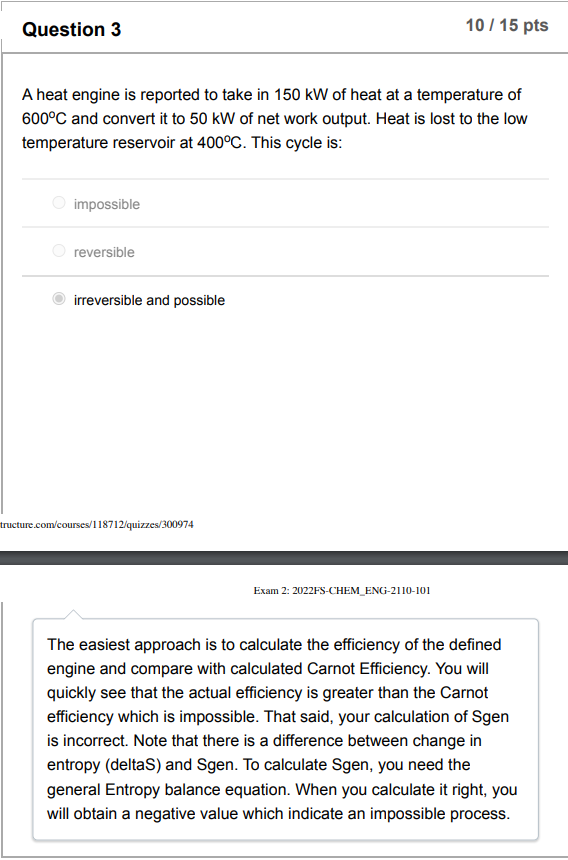
A heat engine is reported to take in 150kW of heat at a temperature of 600C and convert it to 50kW of net work output. Heat is lost to the low temperature reservoir at 400C. This cycle is: impossible reversible irreversible and possible icture.com/courses/118712/quizzes/300974 Exam 2: 2022FS-CHEM_ENG-2110-101 The easiest approach is to calculate the efficiency of the defined engine and compare with calculated Carnot Efficiency. You will quickly see that the actual efficiency is greater than the Carnot efficiency which is impossible. That said, your calculation of Sgen is incorrect. Note that there is a difference between change in entropy (deltaS) and Sgen. To calculate Sgen, you need the general Entropy balance equation. When you calculate it right, you will obtain a negative value which indicate an impossible process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


