Question: please show your work , thank you ! Convert 1.25 moles of Fe2O3 to formula units of Fe2O3. In addition, indicate how many atoms of
please show your work , thank you !
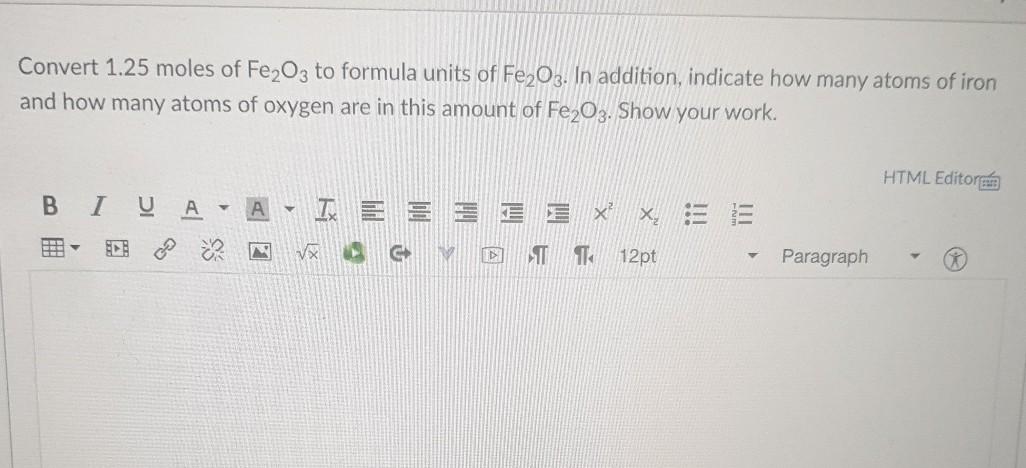
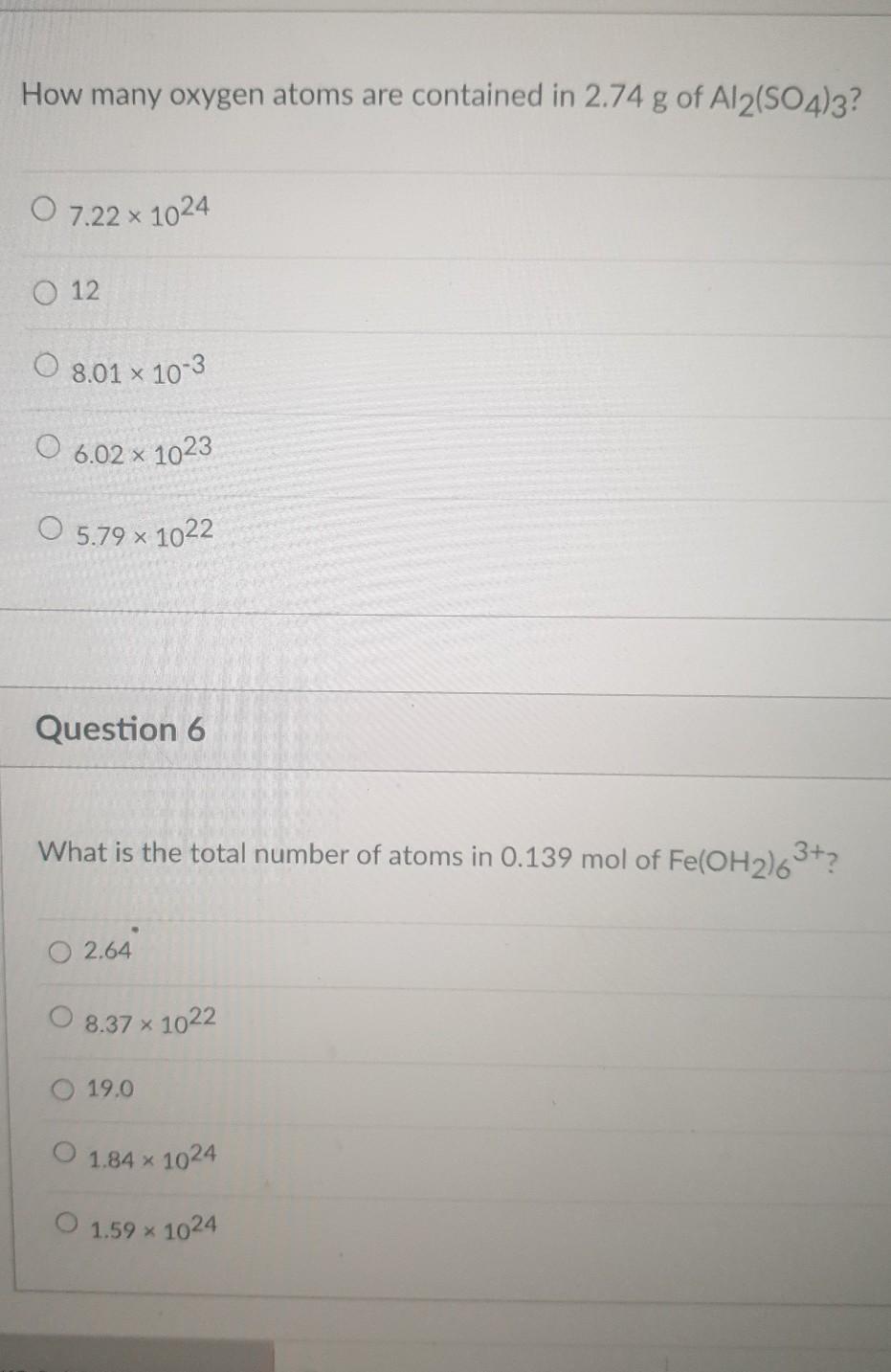
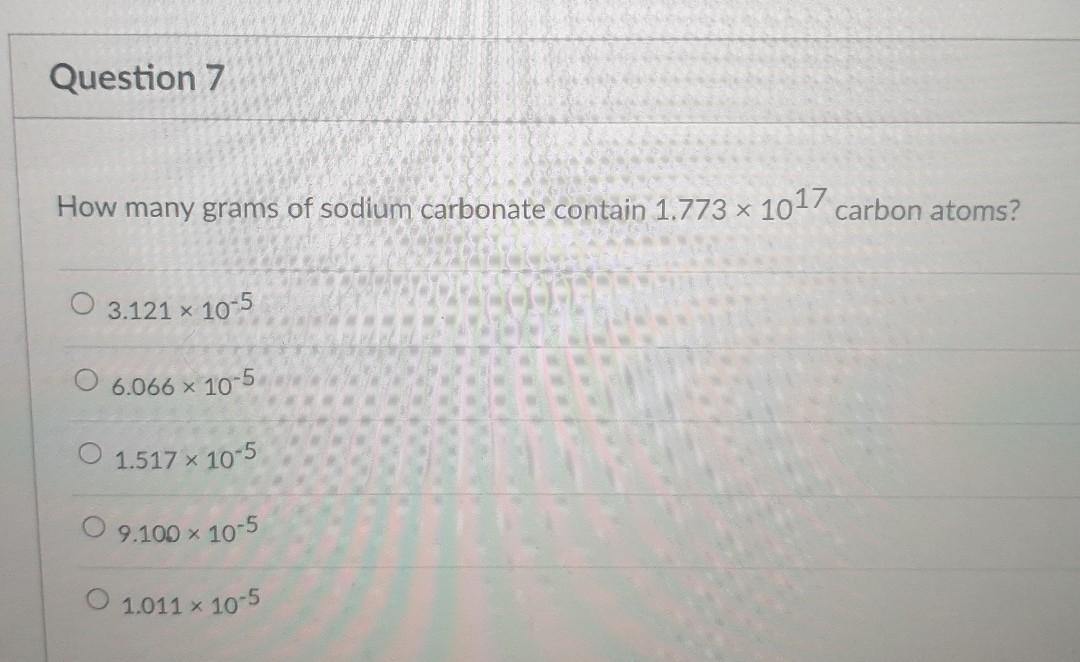
Convert 1.25 moles of Fe2O3 to formula units of Fe2O3. In addition, indicate how many atoms of iron and how many atoms of oxygen are in this amount of Fe2O3. Show your work. HTML Editore IC A IXE E x co 5 2 VX C Moi T TO 12pt Paragraph How many oxygen atoms are contained in 2.74 g of Al2(SO4)3? 0 7.22 x 1024 O 12 O 8.01 x 10-3 O 6.02 x 1023 O 5.79 x 1022 Question 6 What is the total number of atoms in 0.139 mol of Fe(OH2)63+? 0 2.64 8.37 % 1022 19.0 O 1.84 x 1024 O 1.59 X 1024 Question 7 How many grams of sodium carbonate contain 1.773 x 1017 carbon atoms? 3.121 x 10-5 O 6.066 x 10-5 1.517 x 10-5 09.100 x 10-5 O 1.011 x 10-5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


