Question: Naturally occurring samarium includes 15.1% of radioactive isotope 14'Sm, which decays by a emission. One gram of natural Sm gives 89 + 5 a
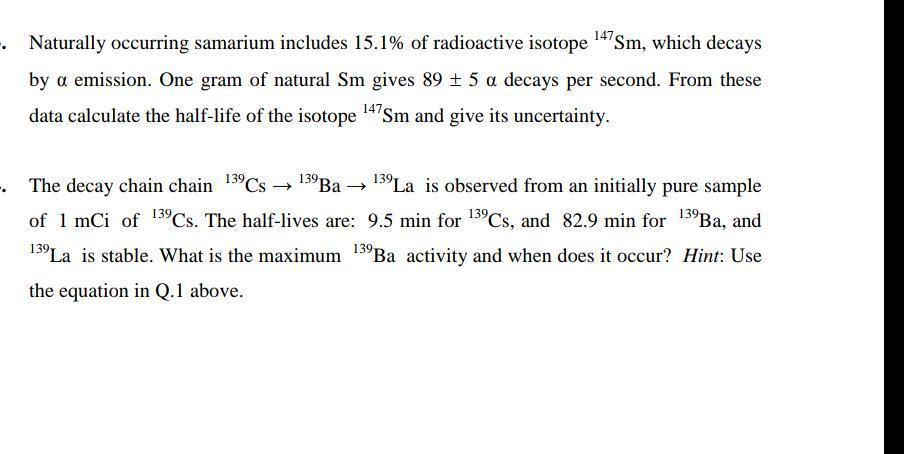
Naturally occurring samarium includes 15.1% of radioactive isotope 14'Sm, which decays by a emission. One gram of natural Sm gives 89 + 5 a decays per second. From these data calculate the half-life of the isotope 14'sm and give its uncertainty. - The decay chain chain 139CS 139Ba 139La is observed from an initially pure sample of 1 mCi of 13CS. The half-lives are: 9.5 min for 1CS, and 82.9 min for 13Ba, and 139 13La is stable. What is the maximum 13Ba activity and when does it occur? Hint: Use the equation in Q.1 above.
Step by Step Solution
3.41 Rating (164 Votes )
There are 3 Steps involved in it
To solve these problems well use the following concepts and equations related to radioactive decay 1 Activity A The rate of decay given by A lambda N where lambda is the decay constant and N is the nu... View full answer

Get step-by-step solutions from verified subject matter experts


