Question: please solve - A solution of HNO, is standardized by reaction with pure sodium carbonate. 2H+ + Na, CO, 2 Na+ + H,0 + CO2
please solve 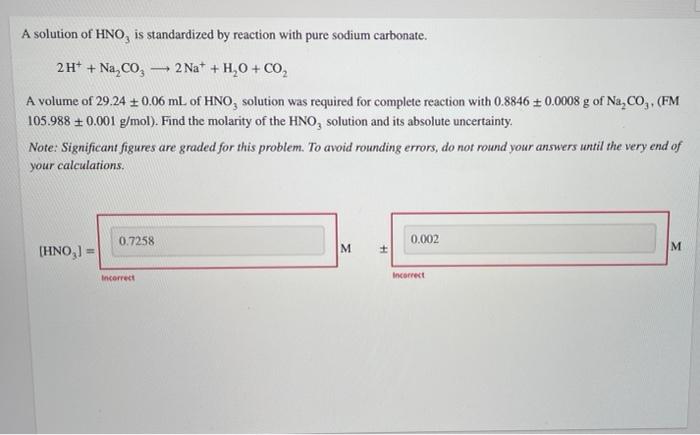

- A solution of HNO, is standardized by reaction with pure sodium carbonate. 2H+ + Na, CO, 2 Na+ + H,0 + CO2 A volume of 29.24 0.06 mL of HNO, solution was required for complete reaction with 0.8846 + 0.0008 g of Na,co (FM 105.988 +0.001 g/mol). Find the molarity of the HNO, solution and its absolute uncertainty. Note: Significant figures are graded for this problem. To avoid rounding errors, do not round your answers until the very end of your calculations. 0.7258 0.002 M M (HNO, - Incorrect Incorrect
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


