Question: Please solve all the options Question 3 (25 pts). Ethanol is produced with the following reaction: C2H4+H2OC2H5OH Fresh feed to a reactor contains 28 mole
Please solve all the options
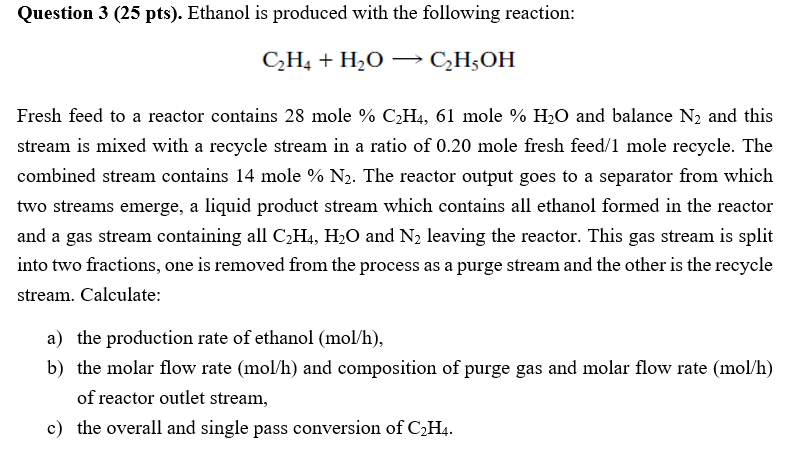
Question 3 (25 pts). Ethanol is produced with the following reaction: C2H4+H2OC2H5OH Fresh feed to a reactor contains 28 mole %C2H4,61 mole %H2O and balance N2 and this stream is mixed with a recycle stream in a ratio of 0.20mole fresh feed /1mole recycle. The combined stream contains 14 mole %N2. The reactor output goes to a separator from which two streams emerge, a liquid product stream which contains all ethanol formed in the reactor and a gas stream containing all C2H4,H2O and N2 leaving the reactor. This gas stream is split into two fractions, one is removed from the process as a purge stream and the other is the recycle stream. Calculate: a) the production rate of ethanol (mol/h), b) the molar flow rate (mol/h) and composition of purge gas and molar flow rate (mol/h) of reactor outlet stream, c) the overall and single pass conversion of C2H4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


