Question: please solve and show all steps and calculation Equilibrium ICE Table II Consider the following reaction for which K=1.60107 at some temperature: 2NOCl(g)2NO(g)+Cl2(g) In a
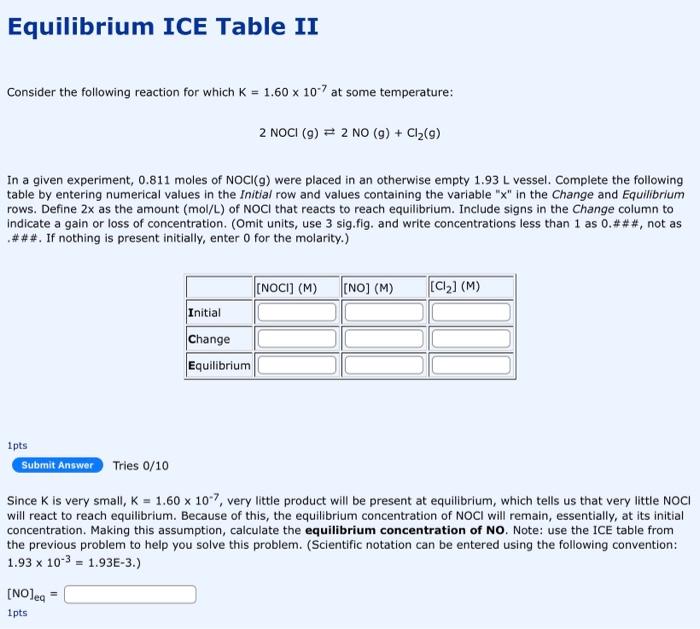
Equilibrium ICE Table II Consider the following reaction for which K=1.60107 at some temperature: 2NOCl(g)2NO(g)+Cl2(g) In a given experiment, 0.811 moles of NOCl(g) were placed in an otherwise empty 1.93L vessel. Complete the following table by entering numerical values in the Initial row and values containing the variable " x " in the Change and Equilibrium rows. Define 2x as the amount (mol/L) of NOCl that reacts to reach equilibrium. Include signs in the Change column to indicate a gain or loss of concentration. (Omit units, use 3 sig.fig. and write concentrations less than 1 as 0 .\#\#\#, not as \#\#\#. If nothing is present initially, enter 0 for the molarity.) 1pts Tries 0/10 Since K is very small, K=1.60107, very little product will be present at equilibrium, which tells us that very little NOCl will react to reach equilibrium. Because of this, the equilibrium concentration of NOCI will remain, essentially, at its initial concentration. Making this assumption, calculate the equilibrium concentration of NO. Note: use the ICE table from the previous problem to help you solve this problem. (Scientific notation can be entered using the following convention: 1.93103=1.93E3. ) [NO]eq= 1 pts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


