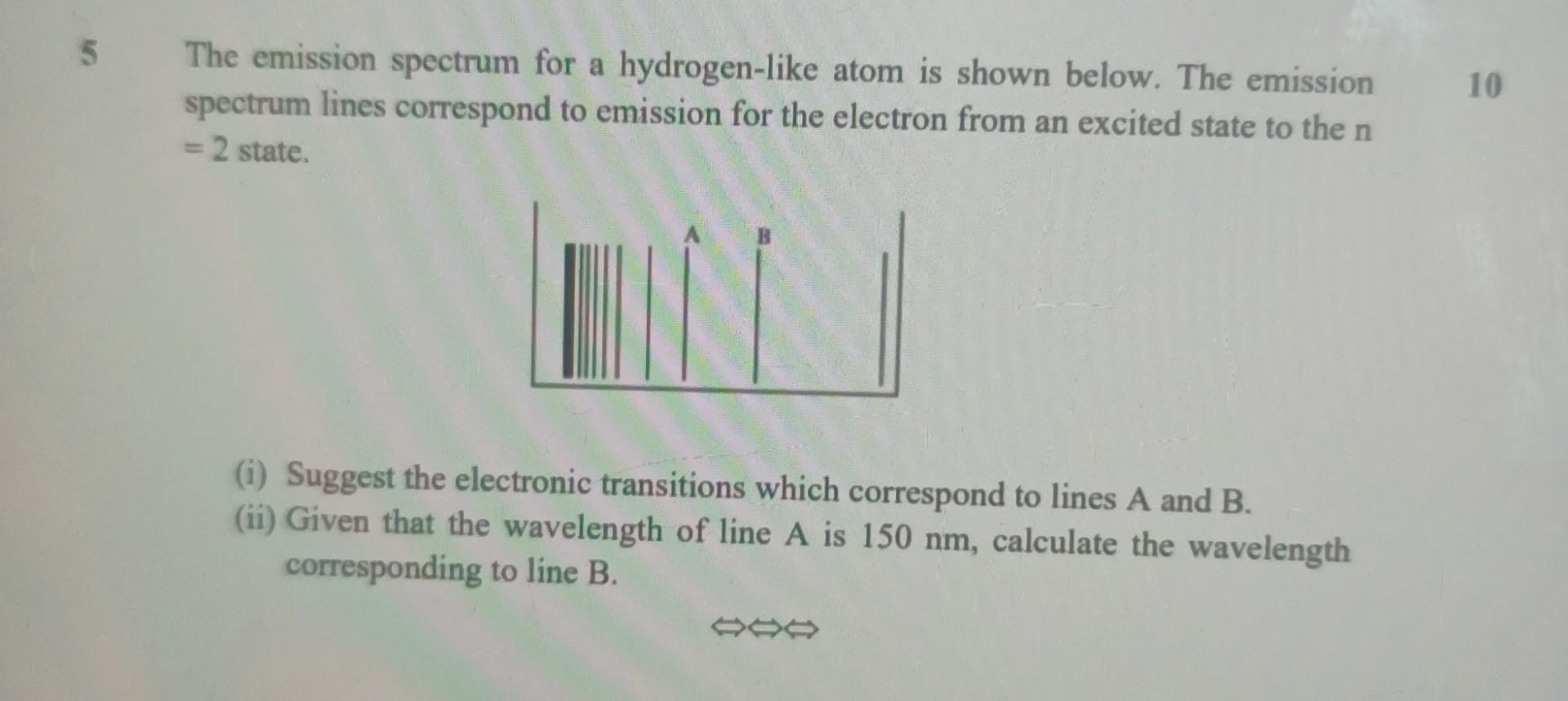
Question: please solve as soon as possible 5 The emission spectrum for a hydrogen-like atom is shown below. The emission spectrum lines correspond to emission for
please solve as soon as possible
5 The emission spectrum for a hydrogen-like atom is shown below. The emission spectrum lines correspond to emission for the electron from an excited state to the n = 2 state. 10 (i) Suggest the electronic transitions which correspond to lines A and B. (ii) Given that the wavelength of line A is 150 nm, calculate the wavelength corresponding to line B
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


