Question: please solve b and c PLEASE 2- The following first order irreversible liquid phase reaction takes place in two equal volume (2m3 of each) CSTRs
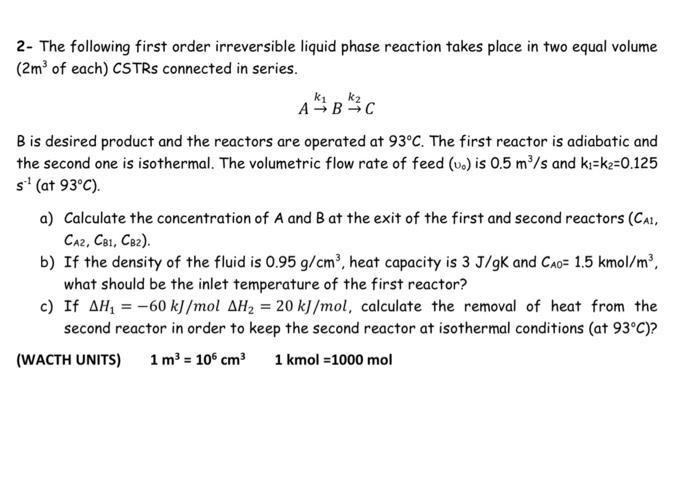
2- The following first order irreversible liquid phase reaction takes place in two equal volume (2m3 of each) CSTRs connected in series. Ak1Bk2C B is desired product and the reactors are operated at 93C. The first reactor is adiabatic and the second one is isothermal. The volumetric flow rate of feed (v0) is 0.5m3/s and k1=k2=0.125 s1( at 93C ). a) Calculate the concentration of A and B at the exit of the first and second reactors (CA1, CA2,CB1,CB2 ). b) If the density of the fluid is 0.95g/cm3, heat capacity is 3J/gK and CA0=1.5kmol/m3, what should be the inlet temperature of the first reactor? c) If H1=60kJ/molH2=20kJ/mol, calculate the removal of heat from the second reactor in order to keep the second reactor at isothermal conditions (at 93C )? (WACTH UNITS) 1m3=106cm31kmol=1000mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


