Question: Please solve biochemistry/ionic equilibrium question. Please give step by step solution. Pyridine (see Appendix C posted in Acid-Base Module) has a Kb of 1.710(9). What
Please solve biochemistry/ionic equilibrium question. Please give step by step solution.
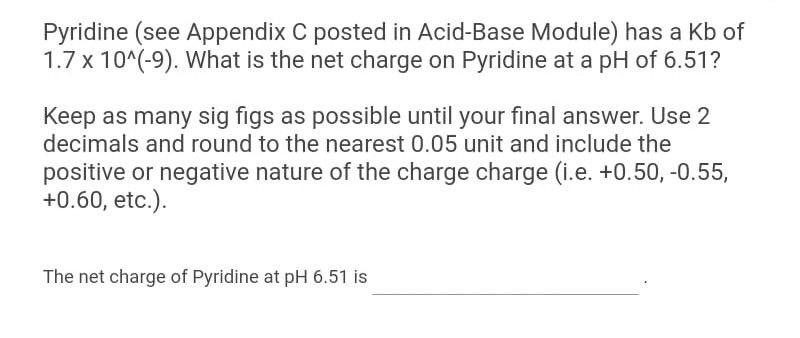
Pyridine (see Appendix C posted in Acid-Base Module) has a Kb of 1.710(9). What is the net charge on Pyridine at a pH of 6.51 ? Keep as many sig figs as possible until your final answer. Use 2 decimals and round to the nearest 0.05 unit and include the positive or negative nature of the charge charge (i.e. +0.50,0.55, +0.60, etc.). The net charge of Pyridine at pH6.51 is
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


