Question: Please solve Clearly with Explanation. Thank you A galvanic cell is at equilibrium (no current flow), consist of Ag and Cu electrodes at standard conditions.
Please solve Clearly with Explanation. Thank you
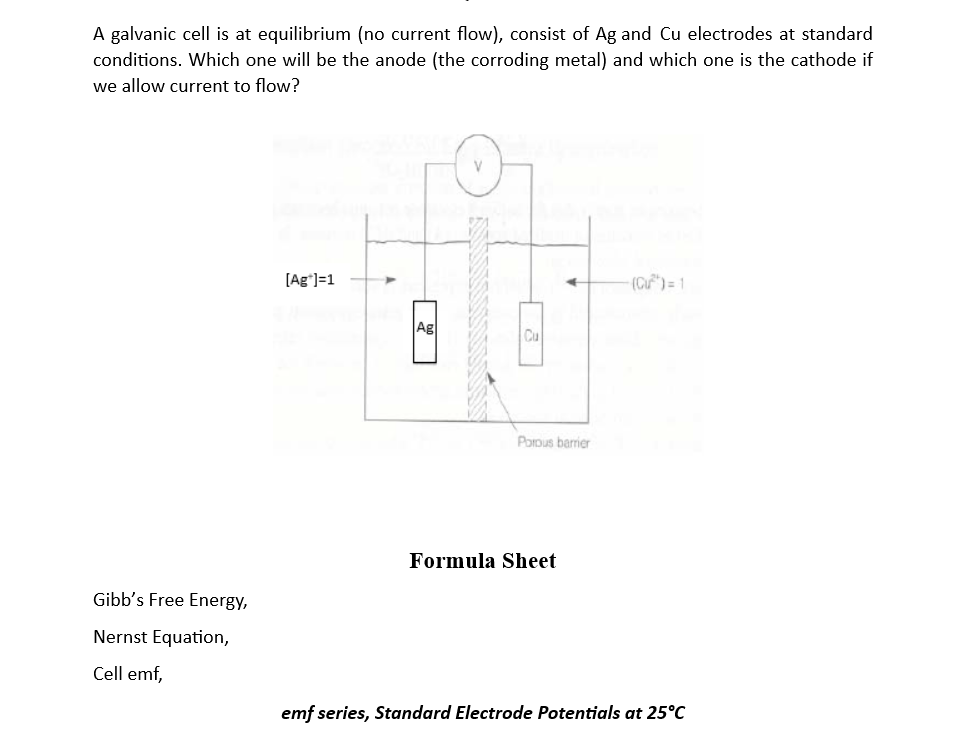
A galvanic cell is at equilibrium (no current flow), consist of Ag and Cu electrodes at standard conditions. Which one will be the anode (the corroding metal) and which one is the cathode if we allow current to flow? [Ag+1=1 (C)=1 Ag) Popus barrier Formula Sheet Gibb's Free Energy, Nernst Equation, Cell emf, emf series, Standard Electrode Potentials at 25C
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


