Question: Please solve Consider the following equilibria and their corresponding equilibrium constants. 2- K] =? C204 (aq) + H (aq) = HC204 (aq) HC,04 (aq) +
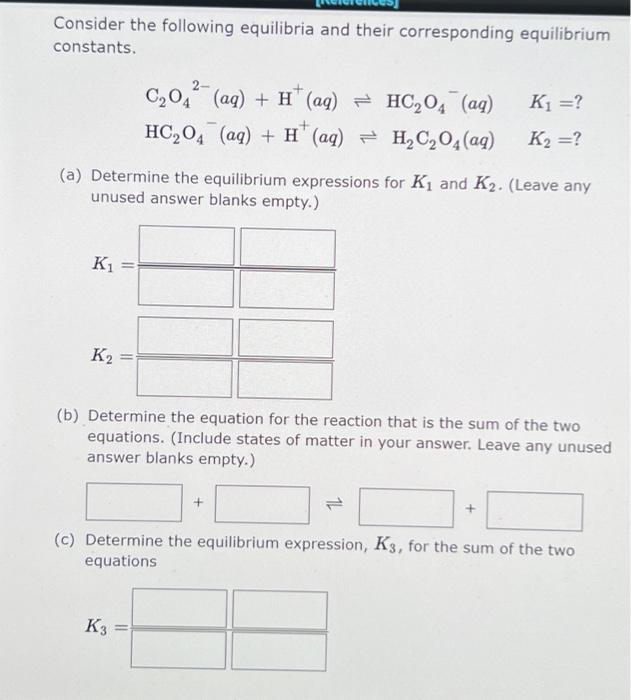
Consider the following equilibria and their corresponding equilibrium constants. 2- K] =? C204 (aq) + H (aq) = HC204 (aq) HC,04 (aq) + H" (aq) = H2C2O4 (aq) K2 =? (a) Determine the equilibrium expressions for Ki and K2. (Leave any unused answer blanks empty.) K1 = K2 (b) Determine the equation for the reaction that is the sum of the two equations. (Include states of matter in your answer. Leave any unused answer blanks empty.) + (C) Determine the equilibrium expression, K3, for the sum of the two equations K3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


