Question: please solve for all parts An aqueous KNO3 solution is made using 60.1g of KNO3 diluted to a total solution volume of 1.96L. (Assurne a
please solve for all parts 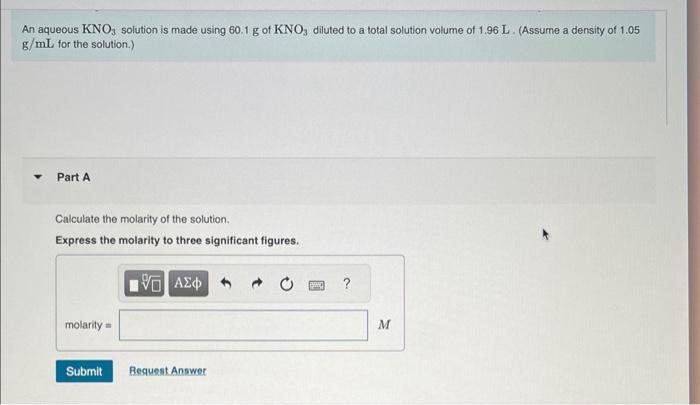

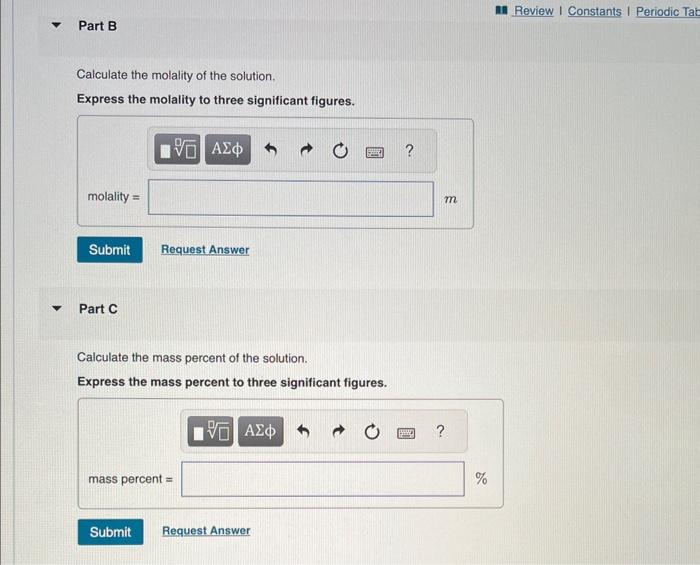
An aqueous KNO3 solution is made using 60.1g of KNO3 diluted to a total solution volume of 1.96L. (Assurne a density of 1.05 g/mL for the solution.) Part A Calculate the molarity of the solution. Express the molarity to three significant figures. Calculate the molality of the solution. Express the molality to three significant figures. Part C Calculate the mass percent of the solution. Express the mass percent to three significant figures
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


