Question: please solve for the activation energy in part C (I've included the correct answers for the previous parts for reference The rate constant of a
please solve for the activation energy in part C (I've included the correct answers for the previous parts for reference 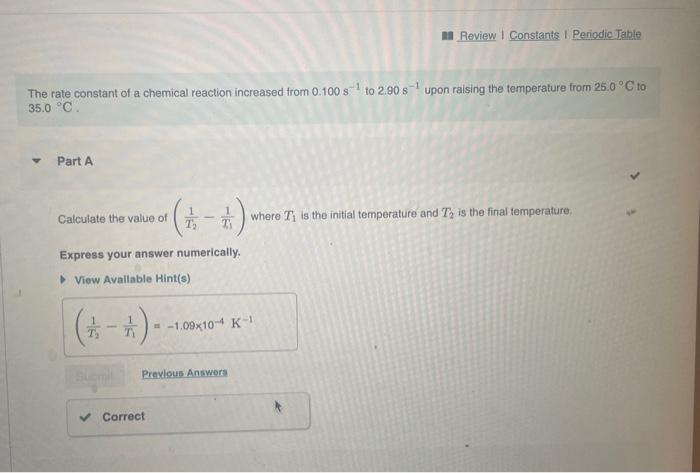

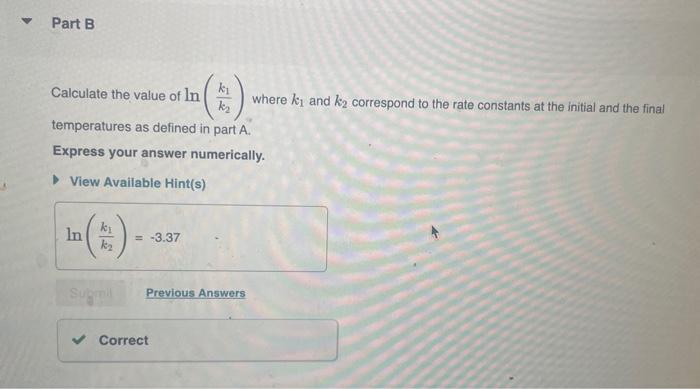
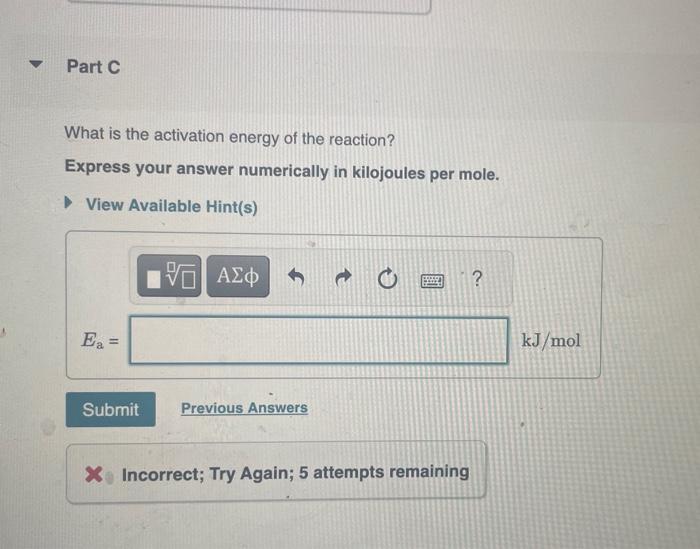
The rate constant of a chemical reaction increased from 0.100s1 to 2.90s1 upon raising the temperature from 25.0C to 35.0C. Part A Calculate the value of (T21T11) where T1 is the initial temperature and T2 is the final temperature. Express your answer numerically. Calculate the value of ln(k2k1) where k1 and k2 correspond to the rate constants at the initial and the final temperatures as defined in part A. Express your answer numerically. What is the activation energy of the reaction? Express your answer numerically in kilojoules per mole
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


