Question: please solve for the reverse reaction and the enthalpy change Current Attempt in Progress The standard heat of the reaction 4NH3(g)+5O2(g)4NO(g)+6H2O(g) Hi=9047kJ Physical Property Tables
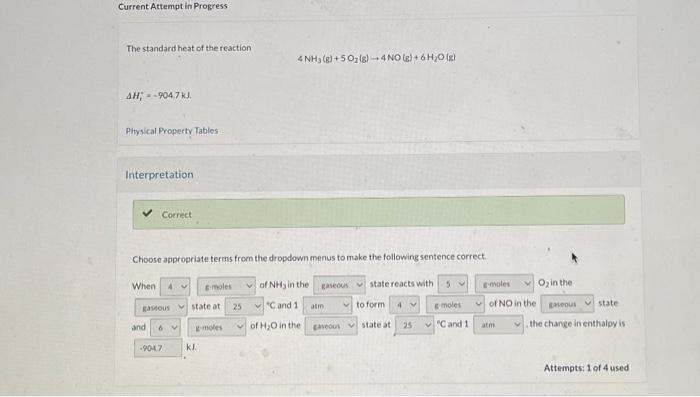
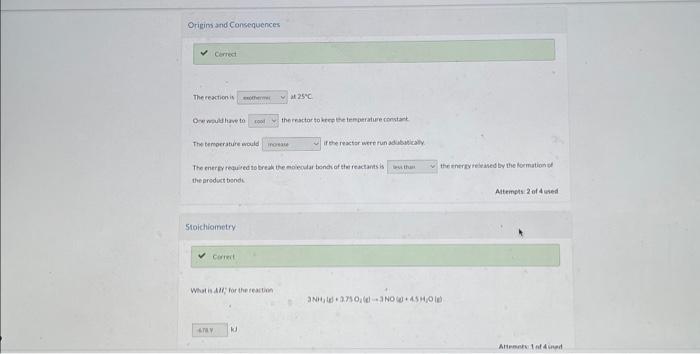
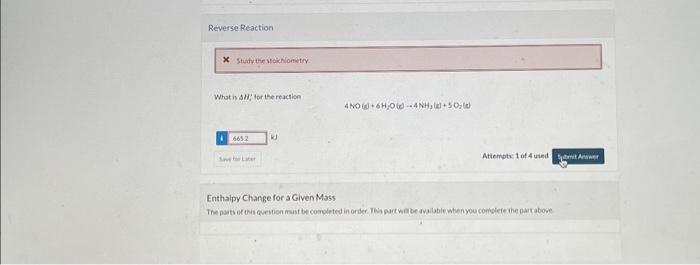
Current Attempt in Progress The standard heat of the reaction 4NH3(g)+5O2(g)4NO(g)+6H2O(g) Hi=9047kJ Physical Property Tables Interpretation Choose appropriate terms from the dropdown menus to make the following sentence correct. kJ. Attempts: 1 of 4 used The reactionis D-e ncild tirve to thereactor fo krep the iringerature rimstart The temoerature equald if the reactsr were fun ad asulealy The enerey reau ed to trea the enolecuar thonct of ster reactants is the inergive iaved by the lormation tod the arodust bonde Altempis 2 of 4 uned 5 toichiometry Whatis ide for the reaction What in $H1 tor the reaction 4NO(g)+6H2O(e)4NH3(e)+5O2(de) Attempts. 1 of 4 uied Enthalpy Change for a Given Mass
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


