Question: Please solve from scratch and do not copy the solution that's already posted for this problem! A mixture of high-temperature gases flows over a horizontal
Please solve from scratch and do not copy the solution that's already posted for this problem!
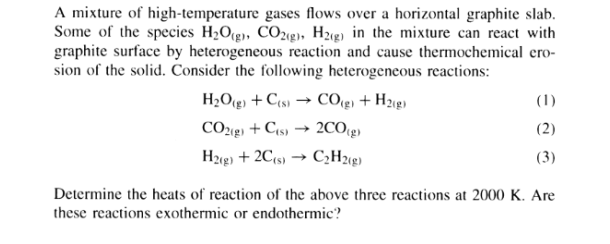
A mixture of high-temperature gases flows over a horizontal graphite slab. Some of the species H2O(g), CO2(g), H2(g) in the mixture can react with graphite surface by heterogeneous reaction and cause thermochemical cro- sion of the solid. Consider the following heterogeneous reactions: H2O(g) + CS CO + H218) (1) CO2(g) + CS 2009) (2) H2(g) + 2Cs) CH2(g) (3) Determine the heats of reaction of the above three reactions at 2000 K. Are these reactions exothermic or endothermic
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


