Question: PLEASE SOLVE IT ASAP WITH THE MATLAB CODES Question 2 - Heat calculations are employed routinely in chemical and bio engineering as well as in
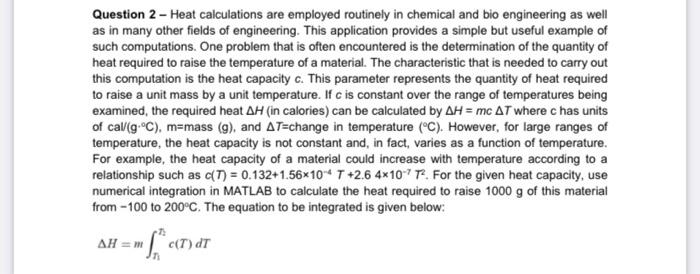
Question 2 - Heat calculations are employed routinely in chemical and bio engineering as well as in many other fields of engineering. This application provides a simple but useful example of such computations. One problem that is often encountered is the determination of the quantity of heat required to raise the temperature of a material. The characteristic that is needed to carry out this computation is the heat capacity c. This parameter represents the quantity of heat required to raise a unit mass by a unit temperature. If c is constant over the range of temperatures being examined, the required heat H (in calories) can be calculated by H=mcT where c has units of cal(gC), mass (g), and T= change in temperature (C). However, for large ranges of temperature, the heat capacity is not constant and, in fact, varies as a function of temperature. For example, the heat capacity of a material could increase with temperature according to a relationship such as c(T)=0.132+1.56104T+2.64107T2. For the given heat capacity, use numerical integration in MATLAB to calculate the heat required to raise 1000g of this material from -100 to 200C. The equation to be integrated is given below: H=mnHc(T)dT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


