Question: please solve it by steps. Question 3: (2 points) The rate of the production of (NH2)C=0 increases by a factor of 4 when the [NH.CNO)
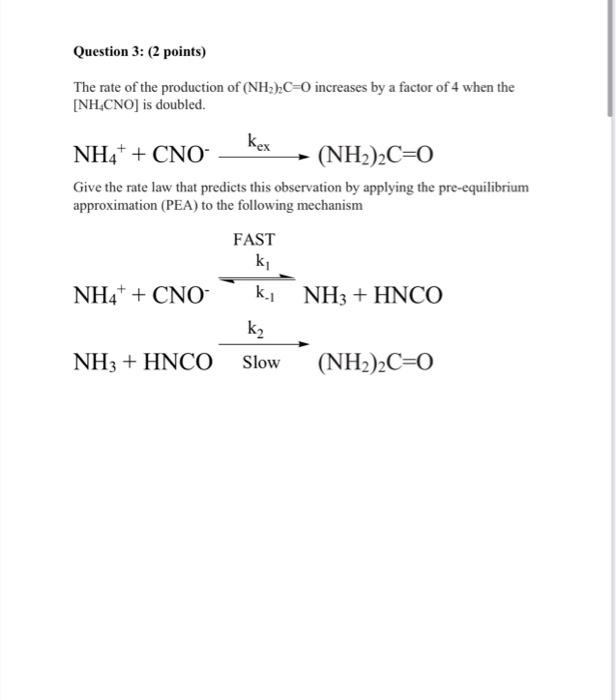
Question 3: (2 points) The rate of the production of (NH2)C=0 increases by a factor of 4 when the [NH.CNO) is doubled. kex NH4+ + CNO (NH2)2C=0 Give the rate law that predicts this observation by applying the pre-equilibrium approximation (PEA) to the following mechanism FAST ki NH4+ + CNO + + ky NH3 + HNCO + k2 NH3 + HNCO Slow (NH2)2C=0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


