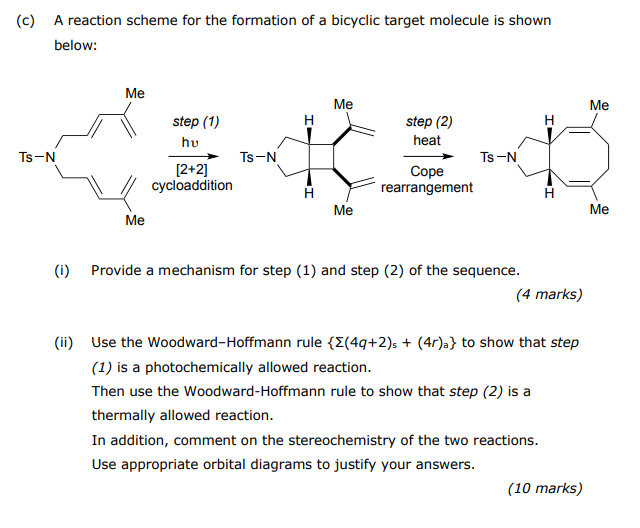
Question: please solve it faster i only have 1 hr (c) A reaction scheme for the formation of a bicyclic target molecule is shown below: Me
please solve it faster i only have 1 hr
(c) A reaction scheme for the formation of a bicyclic target molecule is shown below: Me Me Me H step (2) heat Ts-N step (1) hu [2+2] cycloaddition TS-N Ts-N Cope rearrangement H H Me Me Me (1) Provide a mechanism for step (1) and step (2) of the sequence. (4 marks) (ii) Use the Woodward-Hoffmann rule {E(4q+2)s + (4r)a} to show that step (1) is a photochemically allowed reaction. Then use the Woodward-Hoffmann rule to show that step (2) is a thermally allowed reaction. In addition, comment on the stereochemistry of the two reactions. Use appropriate orbital diagrams to justify your answers. (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


