Question: please solve it step my step and as soon as possible. THANKS what information you need, kindly let me know. assuming each reaction to follow
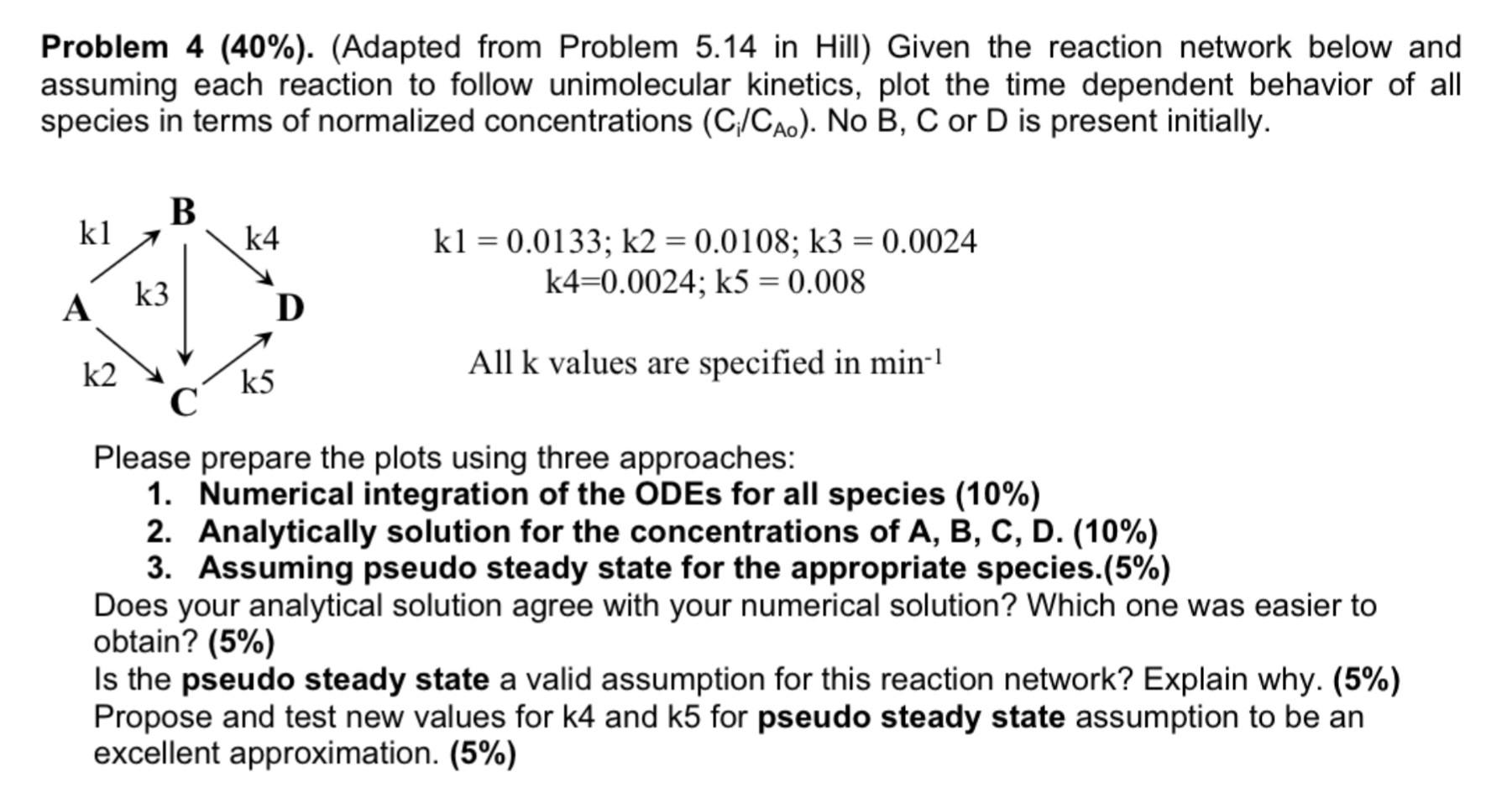
please solve it step my step and as soon as possible. THANKS
what information you need, kindly let me know.
assuming each reaction to follow unimolecular kinetics, plot the time dependent behavior of all species in terms of normalized concentrations (Ci/CA0). No B,C or D is present initially. k1=0.0133;k2=0.0108;k3=0.0024k4=0.0024;k5=0.008 All k values are specified in min1 Please prepare the plots using three approaches: 1. Numerical integration of the ODEs for all species (10%) 2. Analytically solution for the concentrations of A, B, C, D. (10%) 3. Assuming pseudo steady state for the appropriate species.(5\%) Does your analytical solution agree with your numerical solution? Which one was easier to obtain? (5\%) Is the pseudo steady state a valid assumption for this reaction network? Explain why. (5\%) Propose and test new values for k4 and k5 for pseudo steady state assumption to be an excellent approximation. (5\%)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


