Question: please solve it with all steps Table 1: SET B: a) For each given reaction in Table 1, compute the values Gr298 and Hr298 for
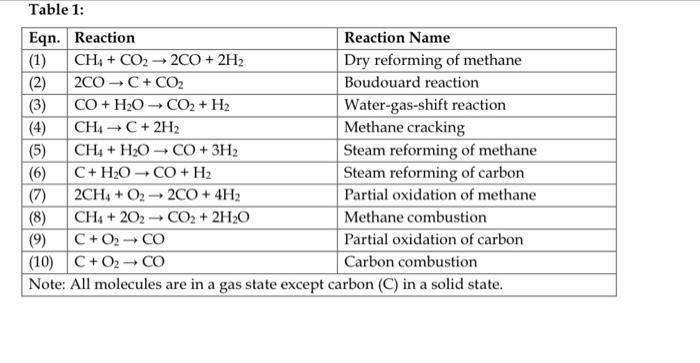
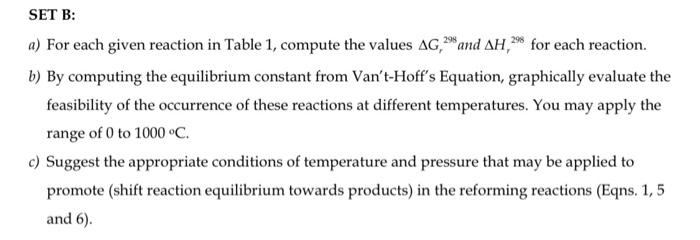
Table 1: SET B: a) For each given reaction in Table 1, compute the values Gr298 and Hr298 for each reaction. b) By computing the equilibrium constant from Van't-Hoff's Equation, graphically evaluate the feasibility of the occurrence of these reactions at different temperatures. You may apply the range of 0 to 1000C. c) Suggest the appropriate conditions of temperature and pressure that may be applied to promote (shift reaction equilibrium towards products) in the reforming reactions (Eqns. 1, 5 and 6)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


