Question: Please solve number 1 (a and b) and number 2. I will upvote if the solution and answer are correct. 1. Suppose a student prepares
Please solve number 1 (a and b) and number 2. I will upvote if the solution and answer are correct.
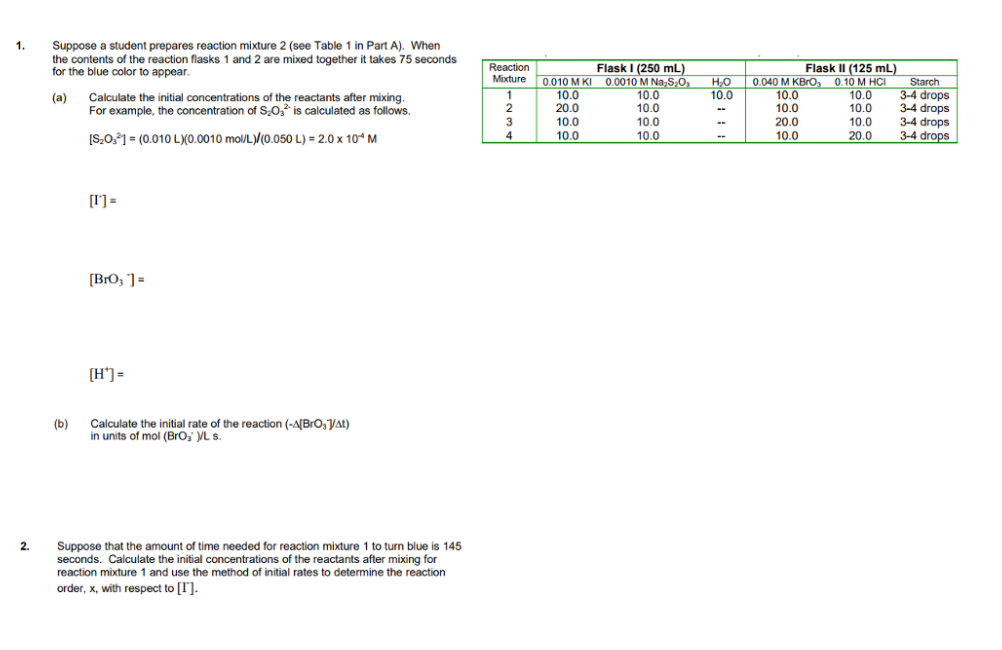
1. Suppose a student prepares reaction mixture 2 (see Table 1 in Part A). When the contents of the reaction flasks 1 and 2 are mixed together it takes 75 seconds for the blue color to appear. (a) Calculate the initial concentrations of the reactants after mixing, For example, the concentration of S.O is calculated as follows. Reaction Mixture 1 1 2 3 4 HO 10.0 Flask 1 (250 ml) 0.010 MKI 0.0010 M N, S,O, 10.0 10.0 20.0 10.0 10.0 10.0 10.0 10.0 Flask II (125 mL) 0.040 M KBrO0.10 M HCI Starch 10.0 10.0 3-4 drops 10.0 10.0 3-4 drops 20.0 10.0 3-4 drops 10.0 20.0 3-4 drops [S2021 = (0.010 LX0.0010 mol/L)/(0.050 L) = 2.0 x 10M [1] - [Bro, ) - [H*) (b) Calculate the initial rate of the reaction (-A[Bro, At) in units of mol (BrOVL S. 2. Suppose that the amount of time needed for reaction mixture 1 to turn blue is 145 seconds. Calculate the initial concentrations of the reactants after mixing for reaction mixture 1 and use the method of initial rates to determine the reaction order, x, with respect to [I]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


