Question: please solve part B. Correct Correct answer is shown. Your answer 0.22 was either rounded differently or used a different number of significant figures than
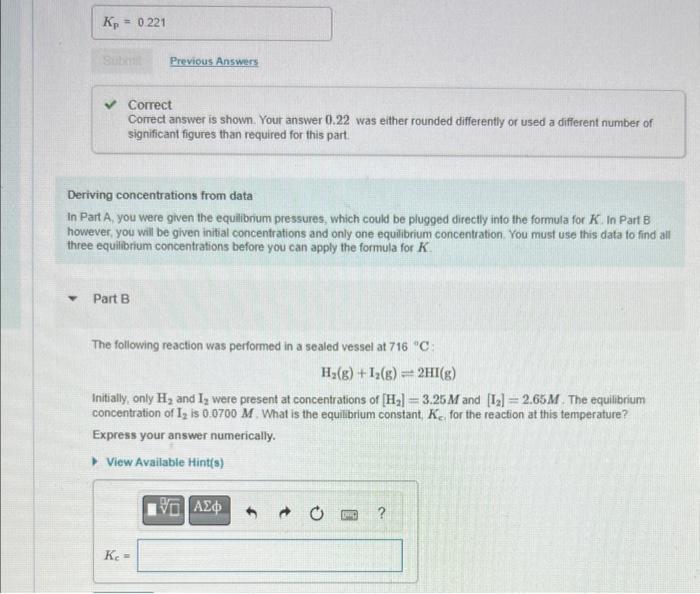
Correct Correct answer is shown. Your answer 0.22 was either rounded differently or used a different number of significant figures than required for this part. Deriving concentrations from data In Part A, you were given the equilibrium pressures, which could be plugged directly into the formula for K. In Part B however, you will be given initial concentrations and only one equilibrium concentration. You must use this data to find all three equilibrium concentrations before you can apply the formula for K. Part B The following reaction was performed in a sealed vessel at 716C : H2(g)+I2(g)2HI(g) Initially, only H2 and I2 were present at concentrations of [H2]=3.25M and [I2]=2.65M. The equilibrium concentration of I2 is 0.0700M. What is the equilbrium constant. Kc for the reaction at this temperature? Express your answer numerically
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


