Question: please solve question question no. 5 .please solve fast. 5. a) Prove that for an ideal gas mixture: Volume% = Pressure% = Mol% b) Calculate
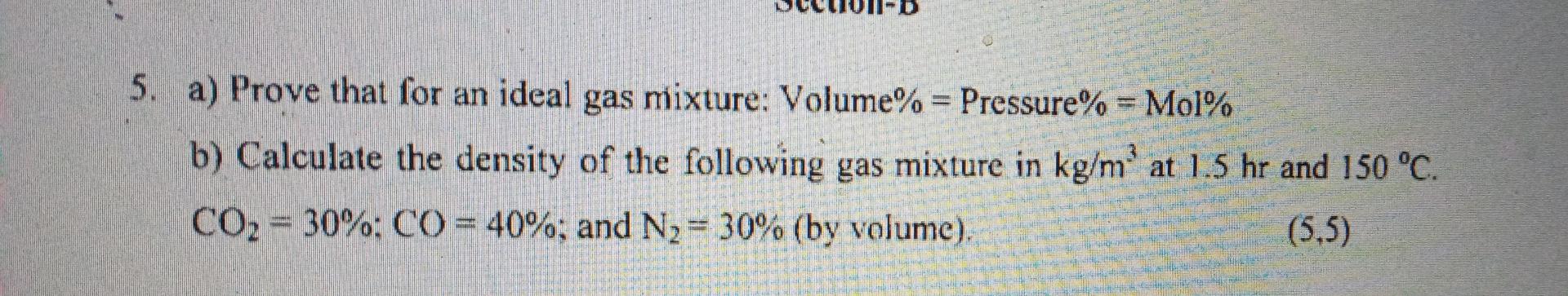
please solve question question no. 5 .please solve fast.
5. a) Prove that for an ideal gas mixture: Volume% = Pressure% = Mol% b) Calculate the density of the following gas mixture in kg/m' at 1.5 hr and 150 C. CO2 = 30%: CO = 40%; and N2 = 30% (by volume). (5,5)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


