Question: Please solve showing full equations. Thanks 6. State whether the reactions are spontaneous or nonspontaneous using the standard reduction potentials? (5 marks) a. CuSO4 (aq)
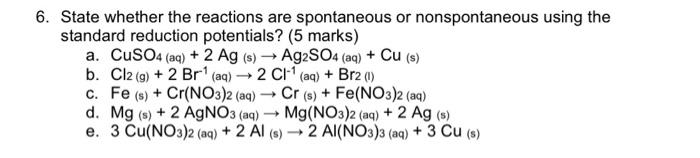
6. State whether the reactions are spontaneous or nonspontaneous using the standard reduction potentials? (5 marks) a. CuSO4 (aq) +2Ag (s) Ag2SO4 (aq) +Cu (s) b. Cl2(g)+2Br1 (aq) 2Cl1 (aq) +Br2 (l) c. Fe (s) +Cr(NO3)2 (aq) Cr (s) +Fe(NO3)2 (aq) d. Mg (s) +2AgNO3 (aq) Mg(NO3)2(aq)+2Ag (s) e. 3Cu(NO3)2(aq)+2Al (s) 2Al(NO3)3 (aq) +3Cu(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


