Question: please solve Starting from the first law, how much liquid water must a 62 kg person evaporate to lower their body temperature by 5'C through
please solve
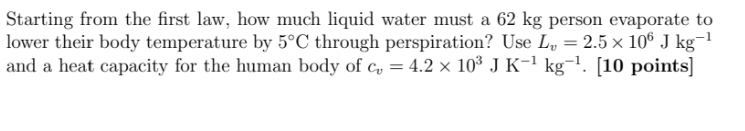
Starting from the first law, how much liquid water must a 62 kg person evaporate to lower their body temperature by 5'C through perspiration? Use Ly = 2.5 x 105 J kg-1 and a heat capacity for the human body of co = 4.2 x 103 J K-kg . [10 points]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


