Question: please solve the calculation part in detail Calculation 1. Calculate the number of moles of ethanol and water in the initial flask, the three distillate

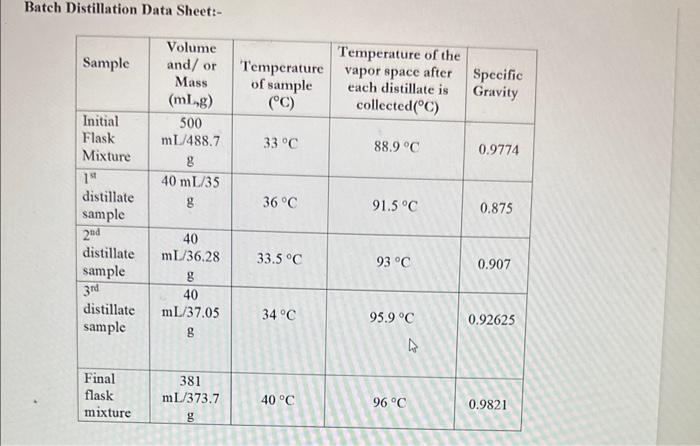

Calculation 1. Calculate the number of moles of ethanol and water in the initial flask, the three distillate samples and the final mixture. Batch Distillation Data Sheet:- Batch Distillation (Lab 05) Concept Distillation separates two or more liquid components in a mixture using the principle of relative volatility or boiling points. The greater the difference in relative volatility the greater the nonlinearity and the easier it is to separate the mixture using distillation. Objectives The objective of this experiment is to apply the process of distillation to a mixture of ethanol and water to obtain a new mixture with an increased concentration of ethanol. In addition, the results of the experiment will be used to approximate the equilibrium relationship between ethanol and water. Specifically, 1. Calculate the number of moles of ethanol and water in the initial flask, the three distillate samples and the final mixture. Calculation 1. Calculate the number of moles of ethanol and water in the initial flask, the three distillate samples and the final mixture. Batch Distillation Data Sheet:- Batch Distillation (Lab 05) Concept Distillation separates two or more liquid components in a mixture using the principle of relative volatility or boiling points. The greater the difference in relative volatility the greater the nonlinearity and the easier it is to separate the mixture using distillation. Objectives The objective of this experiment is to apply the process of distillation to a mixture of ethanol and water to obtain a new mixture with an increased concentration of ethanol. In addition, the results of the experiment will be used to approximate the equilibrium relationship between ethanol and water. Specifically, 1. Calculate the number of moles of ethanol and water in the initial flask, the three distillate samples and the final mixture
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


