Question: Please solve the problem completely and show all necessary work, thank you. Problem 1: A closed, adiabatic, 1000m room contains 60 students. Perform energy balances
Please solve the problem completely and show all necessary work, thank you.
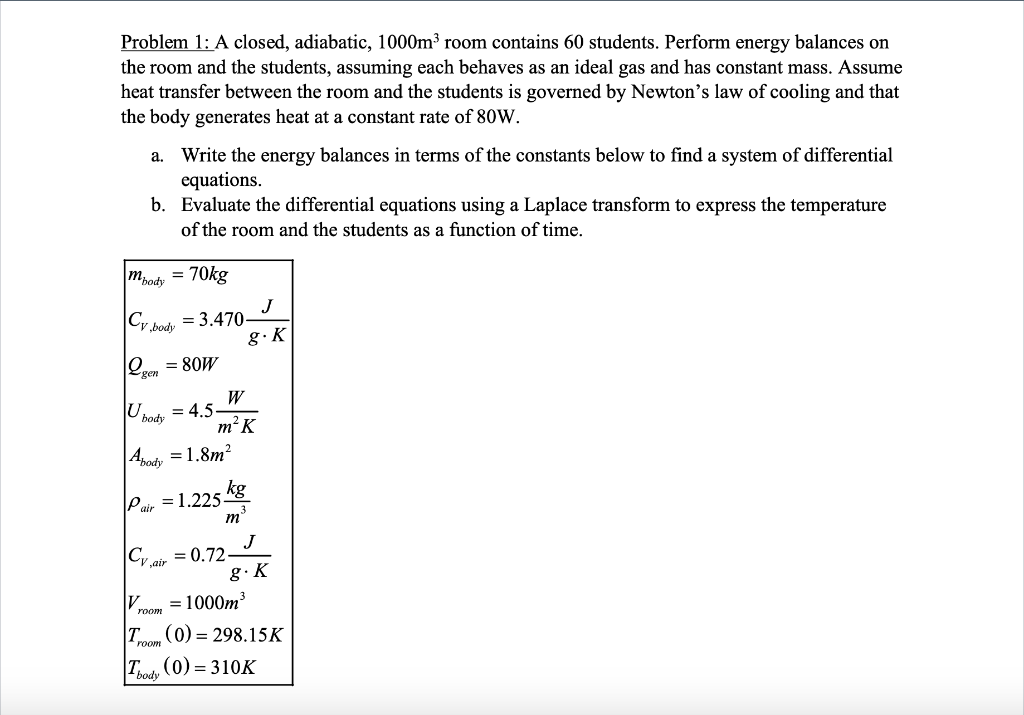
Problem 1: A closed, adiabatic, 1000m room contains 60 students. Perform energy balances on the room and the students, assuming each behaves as an ideal gas and has constant mass. Assume heat transfer between the room and the students is governed by Newton's law of cooling and that the body generates heat at a constant rate of 80W. a. Write the energy balances in terms of the constants below to find a system of differential equations. b. Evaluate the differential equations using a Laplace transform to express the temperature of the room and the students as a function of time. Moody = 70kg J Cr body = 3.470 8.K Q = 80W ge U body W = 4.5 m-K Abody = 1.8m pair = 1.225 kg m J Cy air = 0.72 g.K V = 1000m room T. (0) = 298.15K room Ibody (0) = 310K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


