Question: Please, solve the problem in full explanation and full calculations: One kmol of CO2 is heated at atmospheric pressure to 2900K. What is the equilibrium
Please, solve the problem in full explanation and full calculations:
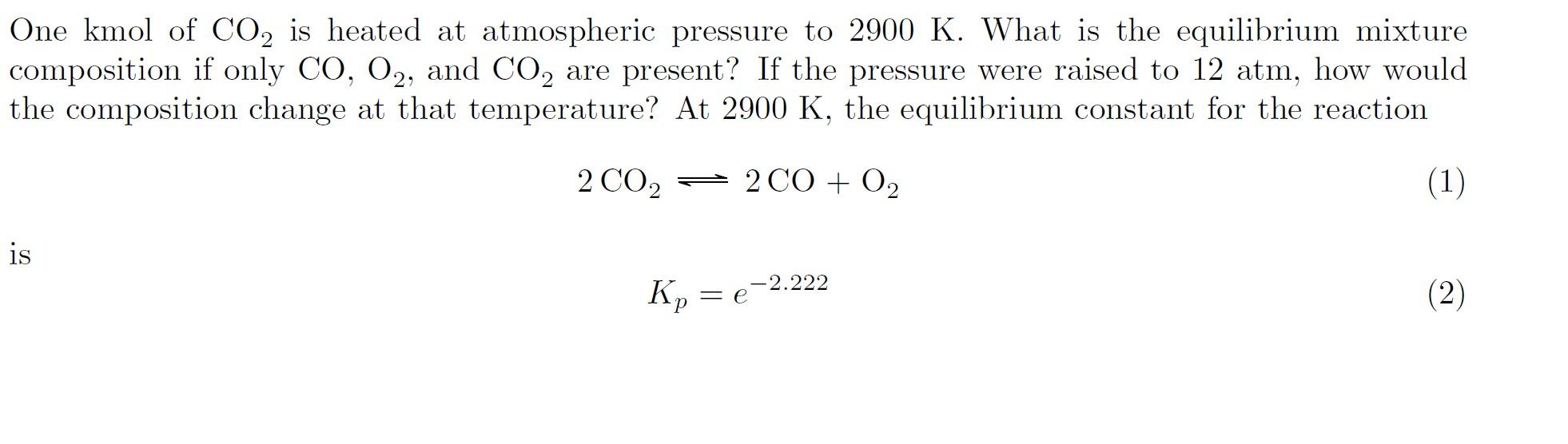
One kmol of CO2 is heated at atmospheric pressure to 2900K. What is the equilibrium mixture composition if only CO,O2, and CO2 are present? If the pressure were raised to 12atm, how would the composition change at that temperature? At 2900K, the equilibrium constant for the reaction 2CO22CO+O2 is Kp=e2.222
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


