Question: please solve them Write complete ionic and net ionic equations for each of the following reactions, HNO3(aq) + KCN(aq) + HCN(g) + KNO3(aq) Instructions B
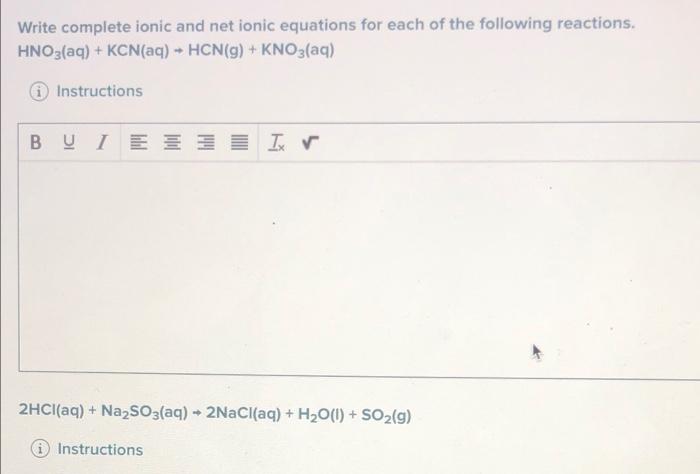


Write complete ionic and net ionic equations for each of the following reactions, HNO3(aq) + KCN(aq) + HCN(g) + KNO3(aq) Instructions B BU I EZ Ix 2HCl(aq) + Na2SO3(aq) + 2NaCl(aq) + H2O(1) + SO2(g) Instructions What is the net ionic equation for the following reaction? HNO3(aq) + K2S(aq) - H2S(g) + KNO3(aq) O A) NO3 (aq) + K (aq) KNO3(aq OB) H(aq) +5+ (aq) - KNO3(aq) "s? O C) NO3(aq) + K (aq) H2S(9) OD) H(aq) + 52 (aq) + H2S(g) The carbon dioxide produced by cells is transported as bicarbonate lons, HCO, What reaction transforms bicarbonate back into carbon dioxide? A) HCO (39+ Hac) + CO3(0) 20g) OB) HCO, (c) HH, CON NG + H (0) = 0,9 ODHCO)* H() - Coa)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


