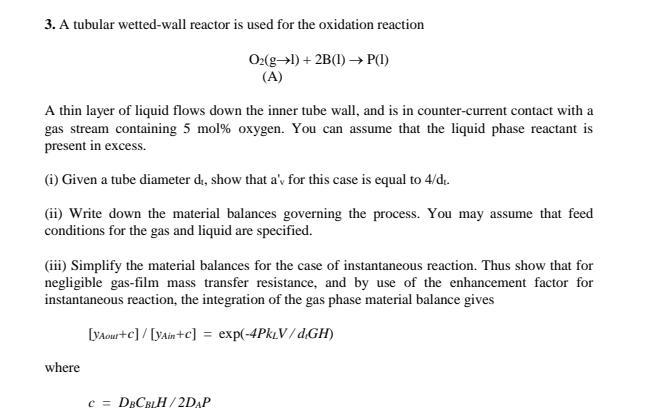
Question: Please solve this as soon as possible Please solve all parts on word 3. A tubular wetted-wall reactor is used for the oxidation reaction O2(gl)+2B(l)P(l)
 Please solve this as soon as possible
Please solve this as soon as possible
Please solve all parts on word
3. A tubular wetted-wall reactor is used for the oxidation reaction O2(gl)+2B(l)P(l) (A) A thin layer of liquid flows down the inner tube wall, and is in counter-current contact with a gas stream containing 5mol% oxygen. You can assume that the liquid phase reactant is present in excess. (i) Given a tube diameter di, show that a'v for this case is equal to 4/dt. (ii) Write down the material balances governing the process. You may assume that feed conditions for the gas and liquid are specified. (iii) Simplify the material balances for the case of instantaneous reaction. Thus show that for negligible gas-film mass transfer resistance, and by use of the enhancement factor for instantaneous reaction, the integration of the gas phase material balance gives [yAour+c]/[yAin+c]=exp(4PkLV/dlGH) where c=DBCBLH/2DAP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


