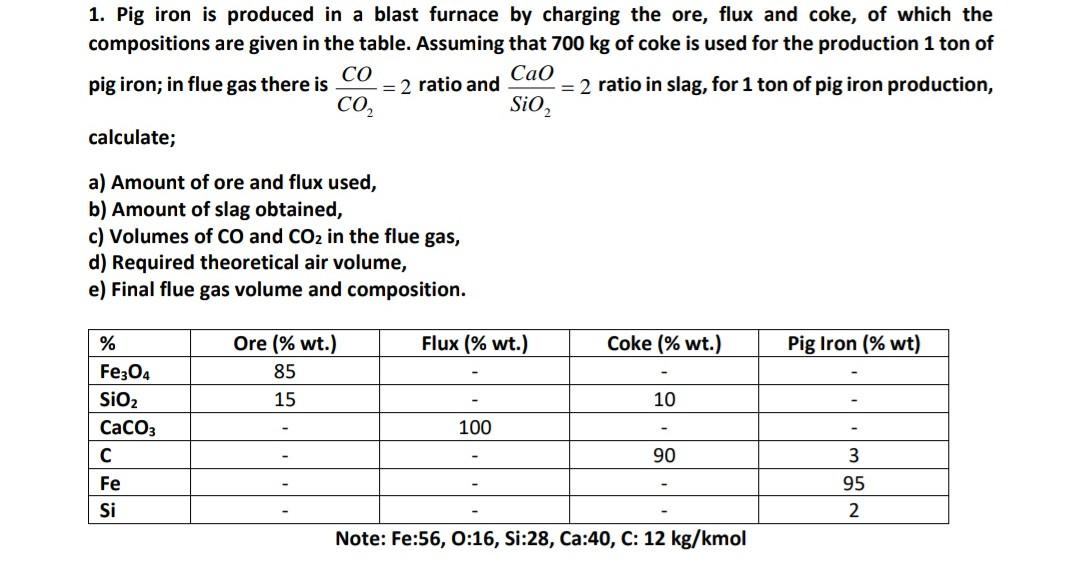
Question: please solve this chemical engineering question (1) 1. Pig iron is produced in a blast furnace by charging the ore, flux and coke, of which
please solve this chemical engineering question (1)
1. Pig iron is produced in a blast furnace by charging the ore, flux and coke, of which the compositions are given in the table. Assuming that 700kg of coke is used for the production 1 to of pig iron; in flue gas there is CO2CO=2 ratio and SiO2CaO=2 ratio in slag, for 1 ton of pig iron production, calculate; a) Amount of ore and flux used, b) Amount of slag obtained, c) Volumes of CO and CO2 in the flue gas, d) Required theoretical air volume, e) Final flue gas volume and composition. Note: Fe:56, 0:16, Si:28, Ca:40, C: 12kg/kmol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


