Question: please solve, this is kinetics A gaseous reactant reacts as follows: Alg) 2RS), (-1A)= kA CA (mol of A/liter-min) The rate constant kA was found
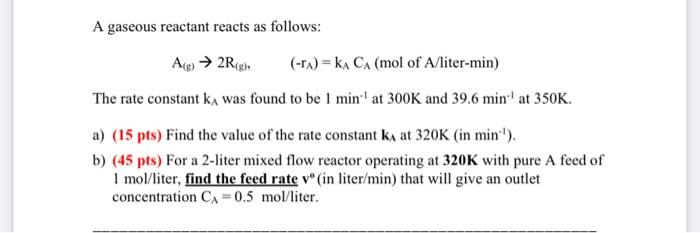
A gaseous reactant reacts as follows: Alg) 2RS), (-1A)= kA CA (mol of A/liter-min) The rate constant kA was found to be 1 min. at 300K and 39.6 min at 350K. a) (15 pts) Find the value of the rate constant ka at 320K (in min:'). b) (45 pts) For a 2-liter mixed flow reactor operating at 320K with pure A feed of 1 mol/liter, find the feed rate v(in liter/min) that will give an outlet concentration CA = 0.5 mol/liter
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


