Question: Please solve for a and b. Please show all steps for both parts (like calculations) not just what equatiins to plug into each other 2.
 Please solve for a and b. Please show all steps for both parts (like calculations) not just what equatiins to plug into each other
Please solve for a and b. Please show all steps for both parts (like calculations) not just what equatiins to plug into each other
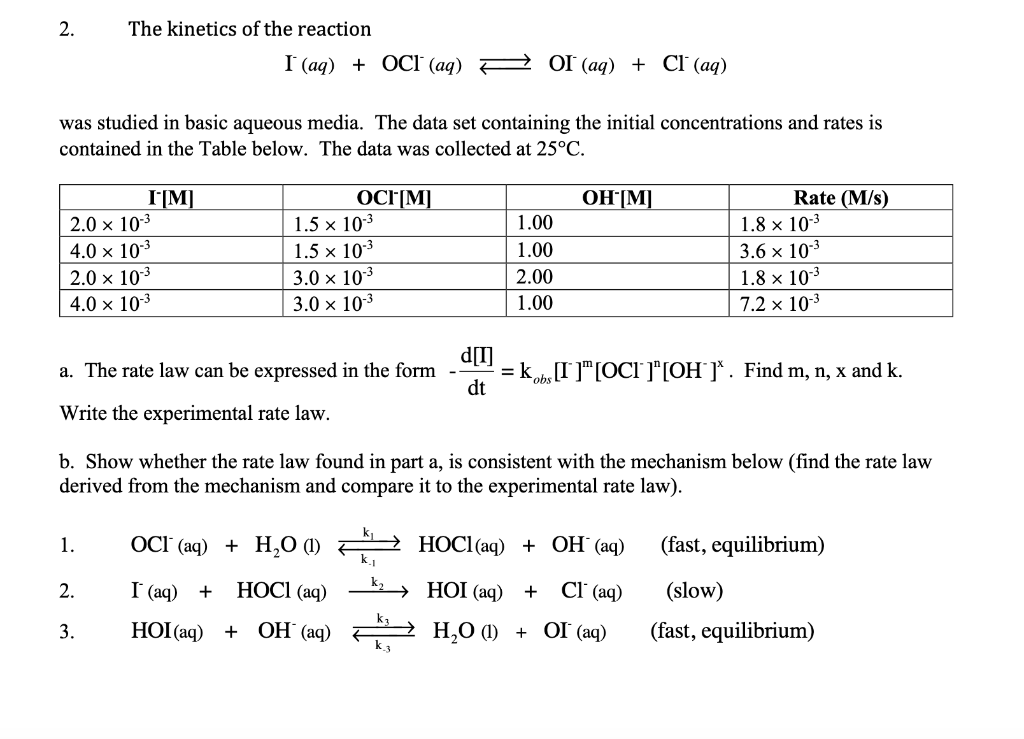
2. The kinetics of the reaction I(aq)+OCl(aq)OI(aq)+Cl(aq) was studied in basic aqueous media. The data set containing the initial concentrations and rates is contained in the Table below. The data was collected at 25C. a. The rate law can be expressed in the form dtd[I]=kobs[I]m[OCl]n[OH]x. Find m,n,x and k. Write the experimental rate law. b. Show whether the rate law found in part a, is consistent with the mechanism below (find the rate law derived from the mechanism and compare it to the experimental rate law). 1. OCl(aq)+H2O (l) k1k1HOCl(aq)+OH(aq) (fast, equilibrium) 2. I(aq)+HOCl(aq)k2HOI(aq)+Cl(aq) (slow) 3. HOI(aq)+OH(aq) k3k3H2O (l) +OI(aq) (fast, equilibrium) 2. The kinetics of the reaction I(aq)+OCl(aq)OI(aq)+Cl(aq) was studied in basic aqueous media. The data set containing the initial concentrations and rates is contained in the Table below. The data was collected at 25C. a. The rate law can be expressed in the form dtd[I]=kobs[I]m[OCl]n[OH]x. Find m,n,x and k. Write the experimental rate law. b. Show whether the rate law found in part a, is consistent with the mechanism below (find the rate law derived from the mechanism and compare it to the experimental rate law). 1. OCl(aq)+H2O (l) k1k1HOCl(aq)+OH(aq) (fast, equilibrium) 2. I(aq)+HOCl(aq)k2HOI(aq)+Cl(aq) (slow) 3. HOI(aq)+OH(aq) k3k3H2O (l) +OI(aq) (fast, equilibrium)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


