Question: please solve this problem and explain how did you do it 5. In a CSTR, the following elementary liquid-phase reaction reaches equilibrium: A+C = 2E
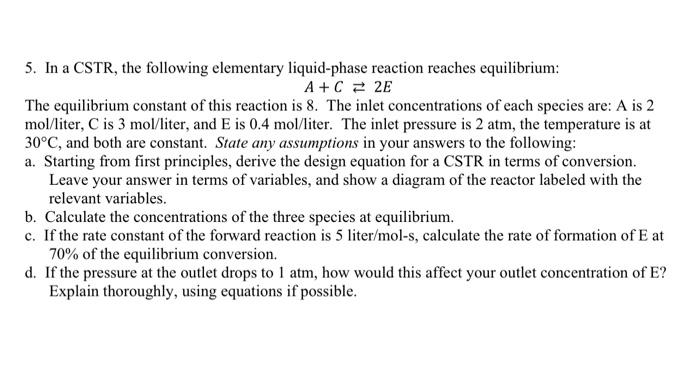
5. In a CSTR, the following elementary liquid-phase reaction reaches equilibrium: A+C = 2E The equilibrium constant of this reaction is 8. The inlet concentrations of each species are: A is 2 mol/liter, C is 3 mol/liter, and E is 0.4 mol/liter. The inlet pressure is 2 atm, the temperature is at 30C, and both are constant. State any assumptions in your answers to the following: a. Starting from first principles, derive the design equation for a CSTR in terms of conversion. Leave your answer in terms of variables, and show a diagram of the reactor labeled with the relevant variables. b. Calculate the concentrations of the three species at equilibrium. c. If the rate constant of the forward reaction is 5 liter/mol-s, calculate the rate of formation of Eat 70% of the equilibrium conversion. d. If the pressure at the outlet drops to 1 atm, how would this affect your outlet concentration of E? Explain thoroughly, using equations if possible
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


